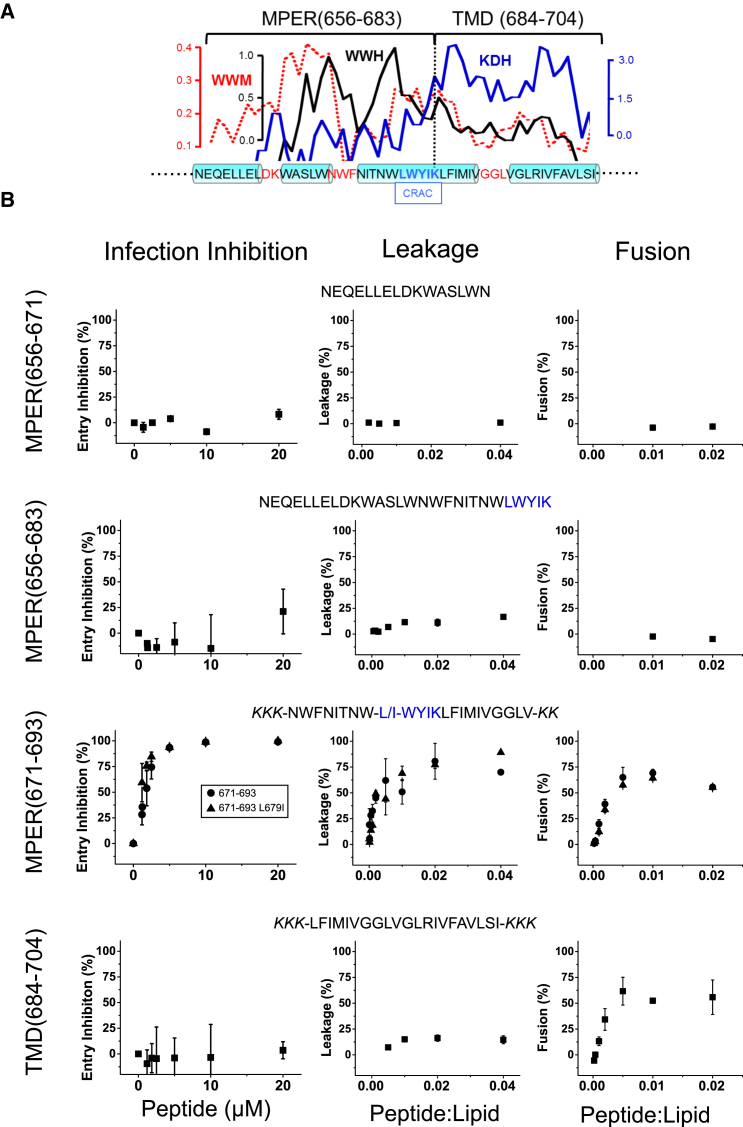
Figure 1.
Designation of hydrophobic-at-interface HIV-1 peptides and their antiviral and membrane-restructuring activities. (A) Given here is the hydrophobicity distribution within the gp41 MPER-TMD region (top) and amino acid sequence (bottom). For calculating mean WW hydrophobicity (33) and KD hydropathy (36), a window of five amino acids was used (WWH and KDH, respectively). The dotted line represents the WW moment (window of 11 amino acids) calculated for a fixed δ = 100° (helical periodicity), using the hydrophobicity-at-interface scale (59). The cylinders on the sequence highlight helical subdomains, which are connected by nonhelical joints. Position for the postulated CRAC motif is also indicated. (B) Shown here are sequences covered by the overlapping peptides used in this study and their effects on pseudovirus infectivity, vesicle permeability, and vesicle-vesicle fusion (left, center, and right panels, respectively). In cell entry inhibition assays, HIV-1 pseudoviruses were preattached to lysine-coated plates (26) and treated with increasing concentrations of the peptides as indicated in the panels. After washing, reporter TZM-bl cells were layered on top, and infectivity inferred from the number of total cells expressing GFP (Fig. S1A). To see this figure in color, go online.