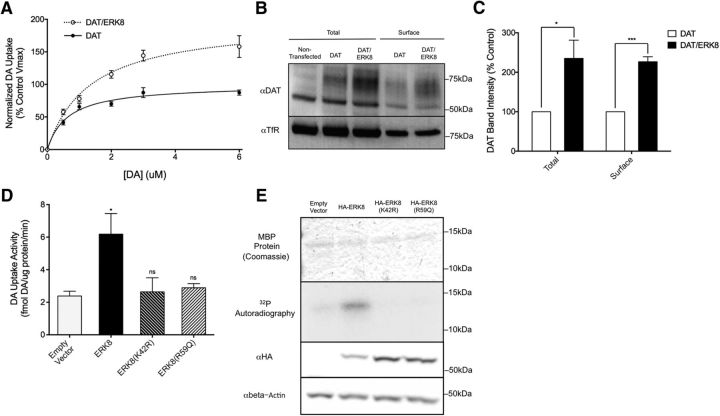
Figure 8.
Human ERK8 regulates DAT activity and protein expression. A, Transfection of human DAT into SH-SY5Y (black line) cells results in saturable, cocaine-sensitive [3H]DA uptake. Cotransfection of ERK8 with DAT (red line) leads to an increase in this [3H]DA uptake, as shown by a ∼98% increase in the Vmax of DA uptake. Data are the average of four experiments, and specific DA uptake was calculated by subtracting DA uptake in the presence of cocaine. In each experiment, DA uptake was normalized to protein levels for each transfection condition, and then each data point was normalized to the VMAX of the DAT transfection alone control from that day, calculated using the Michaelis–Menton equation. Curves were generated using a Michaelis–Menton nonlinear fit in Prism. B, Lysates from SH-SY5Y cells treated with the surface biotinylating agent Sulfo-NHS-biotin were subjected to pull-down using Streptavidin-conjugated beads and eluates from these beads are labeled as “Surface”. Total protein before pulldown was run in parallel (“Total”). Mock transfected cells show no anti-DAT staining at the expected molecular weight of DAT of ∼80 kDa, and DAT/ERK8 transfected cells show a robust increase in labeling compared with DAT transfection alone. A similar increase is seen in surface DAT. Anti-TfR was used as a loading control. C, Quantification of B, total DAT was normalized to TfR and these values were normalized to control DAT transfection alone to generate a percentage control value. For surface levels, surface DAT was normalized to surface TfR, and again values were normalized to control surface levels to generate a percentage control value. ERK8 transfection significantly elevated both total (t(4) = 2.94, p = 0.0425, unpaired t test) and surface DAT (t(4) = 10.02, p = 0.0006, unpaired t test). Data are the average of three experiments and were analyzed using two-tailed Student's t tests for each group of data (total and surface), and a significance threshold was set at p < 0.05. ***p < 0.001. D, Transfection of kinase-dead ERK8(K42R) and vt32-analogous ERK8(R59Q) mutants do not increase DA uptake in SH-SY5Y cells cotransfected with DAT [ERK8(K42R): t(8) = 0.23, p > 0.05, Bonferroni's post-tests; ERK8(R59Q): t(8) = 0.45, p > 0.05, Bonferroni's post-tests]. As previously shown, wild-type ERK8 significantly increases DAT activity (t(8) = 3.40, p < 0.05, Bonferroni's post-tests). Data are the average of three experiments, and were analyzed using a one-way ANOVA with Bonferroni's post-tests comparing each condition to vector control. Significance was set at p < 0.05. *p < 0.01. E, In vitro kinase assay of HA-ERK8, isolated with HA beads, using purified MBP as the substrate. Wild-type HA-ERK8, but neither the kinase-dead HA-ERK8(K42R) nor the HA-ERK8(R59Q) mutant, significantly phosphorylates MBP above background levels. Importantly, all conditions had similar levels of total MBP protein as visualized by Coomassie stain, and ERK8 mutants were expressed at similar levels to WT ERK8, as measured by Western blot analysis of cell lysates using an anti-HA antibody.