Abstract
Venous thromboembolism (VTE) is a common complication in patients with cancer. Previous randomized studies have demonstrated that rates of recurrent VTE are lower in patients treated with low-molecular-weight heparin compared to warfarin. We performed a retrospective analysis of 236 patients with cancer managed by a dedicated oncology anticoagulation management service to compare “real-world” rates of recurrent VTE and bleeding in patients treated with warfarin versus parenteral anticoagulants. Initial anticoagulant regimen included a parenteral agent with transition to warfarin in 132 (55.9%) patients, enoxaparin in 53 (22.5%), dalteparin in 37 (15.7%), and fondaparinux in 14 (5.9%). Taking into account the competing risk of death, cumulative incidence of VTE recurrence at 6 months was 4.0% with warfarin, 10.3% with enoxaparin, 3.0% with dalteparin, and 7.7% with fondaparinux (P = .004). Bleeding complications occurred in 10.6% of patients on warfarin, 17.0% on enoxaprin, 27.0% on dalteparin, and 14.3% on fondaparinux (P = .089). In a dedicated anticoagulation clinic, specific for patients with cancer, warfarin may be an acceptable treatment for first thrombotic events in patients with cancer.
Keywords: anticoagulants, venous thromboembolism, hypercoagulability
INTRODUCTION
The elevated risk of thrombosis in the setting of malignancy has been described for almost 200 years.1 Approximately 20% to 30% of all first venous thromboembolic events (VTE) are related to malignancy, and cancer patients have a relative risk of venous thromboembolism between 4- to 7-fold higher than patients without cancer.2,3 Rates of thromboses in large retrospective studies have been found to be as high as 8% to 12% in some solid tumors types.4,5 Thromboses are more likely in patients with solid tumor having advanced malignancies, specific sites of cancer such as pancreatic, gastric, brain, and gynecologic, and in patients receiving chemotherapy.6 Patients with hematologic malignancies area also at very high risk of thrombosis, with rates between 2% and 10% to 20% for many leukemias, lymphomas, and multiple myelomas.7
Thrombotic events affect morbidity, quality of life and cost of care, and also adversely affect patient mortality.8,9 Treatment of VTE in the patients with cancer is complicated, as the risk of both recurrent thromboembolism and major bleeding is higher than in patients without cancer.10 Patients with malignancy who develop thrombotic events are treated with systemic anticoagulation unless they have an absolute contraindication to anticoagulant therapy. Options for anticoagulation include oral vitamin K antagonists (after bridging with a parenteral agent), subcutaneous low-molecular-weight heparins (LMWHs), such as enoxaparin and dalteparin, and the synthetic pentasaccharide fondaparinux. The largest randomized trial of VTE treatment in patients with active cancer, the CLOT trial, found that dalteparin had greater efficacy than warfarin in preventing recurrent venous thromboembolism.11 The LMWHs are currently recommended for initial treatment of malignancy-associated venous thromboembolism.
Few studies have evaluated the incidence of recurrent thromboses in ambulatory oncology clinics outside the setting of clinical trials. While LMWH is preferred, many patients are not able to be treated with LMWH for a variety of reasons including drug cost and intolerance of or inability to perform injections. Our center has a dedicated anticoagulation management service (AMS) that provides support for clinicians managing patients with cancer, having venous thromboembolism treated with all types of anticoagulants including both warfarin and LMWH.12 We analyzed the rate of recurrent thrombosis in patients with malignancy cared for in this dedicated AMS.
PATIENTS/METHODS
Patient Identification and Eligibility
Following institutional review board approval, we identified patients with active malignancy and a diagnosis of venous thromboembolism managed by the AMS at the Dana-Farber Cancer Institute between January 1, 2008 and January 1, 2012. This patient population reflected patients managed by the AMS since initiation of anticoagulation therapy as well as patients for whom anticoagulation had been started by another provider and were later referred to the AMS. Inclusion criteria included patients 18 years or older, active malignancy - defined as cancer diagnosed or treated within 1 year of the diagnosis of venous thromboembolism - and active anticoagulation - defined as use of an oral or parenteral anticoagulant for at least 1 month after the initial thrombosis.
Data Collection
Chart reviews were performed. Collected patient characteristics included age, gender, type of malignancy, and transplantation status in patients with hematologic malignancy. Information regarding VTE diagnosis included date, method of detection, location, initial anticoagulant regimen, and laboratory values at the time of VTE diagnosis including international normalized ratio (INR), partial thromboplastin time, platelet count, creatinine, alanine aminotransferase, and aspartate aminotransferase. The date and location of recurrent thrombosis, bleeding events, laboratory values at the time of these events, and patient outcomes including date and cause of death or last known follow-up were recorded. A bleeding event was defined as a composite result of major and clinically relevant nonmajor bleeds. Major bleeding was defined as overt bleeding that led to transfusion of red cells, occurred in a critical site, or contributed to death. Clinically relevant nonmajor bleeding was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with the need for hospitalization, medical intervention, or interruption or discontinuation of anticoagulation.
Statistical Analysis
The primary aim was to compare the rate of first recurrent venous thromboembolic event in patients with cancer treated with warfarin versus a parenteral agent. The cumulative incidence rate of recurrent VTE was assessed by a competing risk analysis. Time to first recurrent VTE during the first anticoagulant regimen was measured from the time of diagnosis of the first clot to the first subsequent VTE, death, or termination of the first anticoagulant regimen, whichever occurred first. Death before recurrent VTE while on the first anticoagulant agent was considered as a competing risk event. Patients alive and without a recurrent VTE at the end of the first anticoagulant regimen were censored at the time anticoagulation was discontinued. Reasons for stopping the first anticoagulant include recurrent VTE, death, bleeding event, or were unknown based on information available. Differences in the incidence rate of recurrent VTE on different anticoagulant agents were evaluated by the Gray test, and the Fine and Gray regression model was used to assess the impact of the first anticoagulant regimen on the time to first recurrent VTE. Other covariates were similarly assessed. The number and proportion of patients with a recurrent VTE while on first anticoagulant is also provided along with the corresponding incidence rate calculated per person-time.
Overall survival (OS), defined as time from diagnosis of the first VTE to date of death from any cause, was estimated using the method of Kaplan and Meier and the log-rank test was used to compare OS between groups. Patients alive at the end of the follow-up period were censored at date of last known contact. Univariate logistic regression modeling was used to assess the impact of the first anticoagulant regimen on the probability of a bleeding episode on that regimen. Other covariates were also assessed using univariate logistic regressions.
Patient characteristics were summarized using proportions and ranges for categorical end points and medians for continuous end points. Fisher exact test and the Kruskal-Wallis test were used to compare categorical and continuous variables among groups, respectively. Statistical significance was defined as a P value <.05. Statistical analyses were performed using SAS statistical software (version 9.3, SAS institute) and the cmprsk package of R.
RESULTS
Baseline Characteristics
A total of 239 patients met the inclusion criteria. Three patients whose initial anticoagulant agent was either unknown (1 patient) or none (2 patients) after the first VTE were excluded from the analyses; data from 236 patients was included. The baseline characteristics of these patients are shown in Table 1. The median age was 61.7 years (range 19.5–88.9) and 100 (42.4%) patients were male. Of the patients, 169 (71.6%) had solid malignancies and 67 (28.4%) had hematologic malignancies. In patients with solid malignancies, breast (30 patients, 17.8%), colorectal (24 patients, 14.3%), and sarcoma (17 patients, 10.1%) were the most common. Ninety-eight (58%) patients with solid malignancy had metastatic disease. In patients with hematologic malignancies, non-Hodgkin lymphoma and multiple myeloma (22 patients, 32.8% for each type) were the most common. Of the patients with hematologic malignancy, 32 (47.8%) underwent hematopoietic stem cell transplantation either before study entry or during the time they were included in the study.
Table 1.
Baseline Characteristics.
| Age at first clot, median (range) | 61.7 (19.5–88.9) |
| Gender | |
| Female | 136 (57.6%) |
| Male | 100 (42.4%) |
| Solid Malignancy, n (%) | 169 (71.6%) |
| Breast | 30 (17.8%) |
| Colorectal | 24 (14.3%) |
| Sarcoma | 17 (10.1%) |
| Lung | 13 (7.7%) |
| Ovarian | 13 (7.7%) |
| Bladder | 12 (7.1%) |
| Head and neck | 9 (5.3%) |
| Other | 51 (30.2%) |
| Solid stage, n (%) | |
| Metastatic | 98 (58.0%) |
| Localized | 71 (42.0%) |
| Hematologic malignancy, n (%) | 67 (28.4%) |
| Non-Hodgkins lymphoma | 22 (32.8%) |
| Multiple myeloma | 22 (32.8%) |
| Hodgkins lymphoma | 6 (9.0%) |
| Chronic lymphocytic leukemia | 6 (9.0%) |
| Acute myeloid leukemia | 5 (7.5%) |
| Other | 6 (9.0%) |
| Transplant (hematologic), n (%) | 32 (47.8%) |
| Initial clot location | |
| Deep vein thrombosis (DVT) | 122 (51.7%) |
| Pulmonary embolism (PE) | 69 (29.2%) |
| DVT and PE | 22 (9.3%) |
| Inferior vena cava (IVC) | 8 (3.4%) |
| Portal vein | 4 (1.7%) |
| Intracardiac | 3 (1.3%) |
| Intracranial Vein | 3 (1.3%) |
| Gonadal | 2 (0.8%) |
| Other | 3 (1.3%) |
| Initial therapy, n (%) | |
| Warfarin | 132 (55.9%) |
| Enoxaparin | 53 (22.5%) |
| Dalteparin | 37 (15.7%) |
| Fondaparinux | 14 (5.9%) |
Venous Thromboembolism Type and Initial Management
In all, 122 (51.7%) patients had deep venous thrombosis (DVT), 69 (29.2%) had pulmonary embolism (PE), 22 (9.3%) had both DVT and PE, and 23 (9.7%) had thrombus in other locations including the portal vein, gonadal vein, intracranial vein, and intracardiac thrombosis. Initial anticoagulant agents included warfarin in 132 (55.9%) patients, enoxaparin in 53 (22.5%), dalteparin in 37 (15.7%), and fondaparinux in 14 (5.9%). The distribution of patient characteristics by type of first anticoagulation is shown in Table 2. Age at first clot, patient gender, malignancy type, stage of solid malignancy (localized versus metastatic), transplantation status (for hematologic malignancies), initial clot location, and whether a patient was on chemotherapy at the time of the first VTE did not differ significantly between different anticoagulation regimens. The time in therapeutic range (TTR) at our center for patients on warfarin was 59.5% during the study time period.
Table 2.
Patient Characteristics and Type of First Anticoagulation.
| Warfarin (N = 132) | Enoxaparin (N = 53) | Dalteparin (N = 37) | Fondaparinux (N = 14) | P Valuea | |
|---|---|---|---|---|---|
| Age at first clot, median (range) | 61.2 (19.5–88.8) | 64.2 (25.3–85.4) | 61.5 (40.1–85.7) | 61.1 (49.0–83.2) | .3887 |
| Gender, n (%) | .7301 | ||||
| Male | 53 (40.2%) | 26 (49.1%) | 15 (40.5%) | 6 (42.9%) | |
| Female | 79 (59.9%) | 27 (50.9%) | 22 (59.5%) | 8 (57.1%) | |
| Initial clot location, n (%) | .7513 | ||||
| DVT | 73 (55.3%) | 24 (45.3%) | 18 (48.7%) | 7 (50%) | |
| PE | 32 (24.2%) | 21 (39.6%) | 11 (29.7%) | 5 (35.7%) | |
| DVT and PE | 12 (9.1%) | 4 (7.6%) | 5 (13.5%) | 1 (7.1%) | |
| Other | 15 (11.4%) | 4 (7.6%) | 3 (8.1%) | 1 (7.1%) | |
| Malignancy type, n (%) | .1663 | ||||
| Solid | 90 (68.2%) | 36 (67.9%) | 31 (83.8%) | 12 (85.7%) | |
| Hematologic | 42 (31.8%) | 17 (32.1%) | 6 (16.2%) | 2 (14.3%) | |
| Solid stage, n (%) | |||||
| Localized | 41 (45.5%) | 17 (47.2%) | 9 (29%) | 4 (33.3%) | |
| Metastatic | 49 (54.4%) | 19 (52.8%) | 22 (71%) | 8 (67.7%) | |
| Transplant (hematologic), n (%) | .2079 | ||||
| Yes | 17 (40.5%) | 9 (52.9%) | 5 (83.3%) | 1 (50%) | |
| No | 25 (59.5%) | 3 (47.1%) | 1 (16.7%) | 1 (50%) | |
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism.
P value from Kruskal-Wallis test for age, chi-squared for clot location, and Fisher Exact for all other parameters.
Recurrent Venous Thromboembolism while on First Anticoagulant Regimen
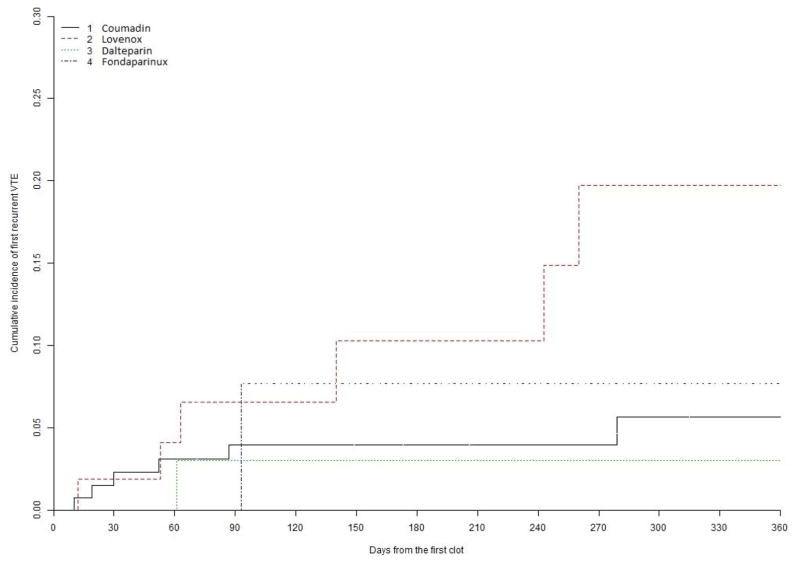
Taking into account the competing risk of death, cumulative incidence of VTE recurrence while on first anticoagulant at 6 months was 4.0% on warfarin, 10.3% on enoxaparin, 3.0% on dalteparin, and 7.7% on fondaparinux (Gray test P = .004). This is shown in Table 3 and Figure 1. Patients on enoxaparin were significantly more likely to develop recurrent VTE than those on warfarin (hazard ratio [HR] 4.65, 90% confidence interval [CI] 1.98–10.96, P value .003). The risk of recurrent VTE did not differ significantly between dalteparin and warfarin (HR 0.562, 90% CI 0.096–3.290) or fondaparinux and warfarin (HR 1.23, 90% CI 0.206–7.360). Wald test showed a significant difference in recurrent VTE by regimen when all 4 regimens were compared (P = .013), likely driven by the higher rate of recurrence in patients on enoxaparin. Age, gender, malignancy type, transplantation status, or initial clot location did not significantly impact the risk of recurrence.
Table 3.
Cumulative Incidence of Recurrent VTE During First Anticoagulant by Type of Initial Anticoagulation.
| Anticoagulant Regimen | Patients on Regimen | Recurrent VTE, # (%) | 3-Month VTE Recurrence, (%) | 6-Month VTE Recurrence, (%) | Incidence rate (per 1000 person-months) |
|---|---|---|---|---|---|
| Warfarin | 132 | 7 (5.3%) | 4.0% | 4.0% | 4.0 |
| Enoxaparin | 53 | 9 (17.0%) | 6.6% | 10.3% | 24.4 |
| Dalteparin | 37 | 1 (2.7%) | 3.0% | 3.0% | 2.6 |
| Fondaparinux | 14 | 1 (7.1%) | 0.0% | 7.7% | 5.0 |
| Gray test | 0.004 |
Abbreviation: VTE, venous thromboembolism.
Figure 1.
Incidence of recurrent thrombosis by anticoagulant regimen.
Bleeding Events while on First Anticoagulant Regimen
Of 132 patients, 14 (10.6%) developed bleeding events while on warfarin, compared to 9 (17.0%) of 53 patients on enoxaparin, 10 (27.0%) of 37 patients on dalteparin, and 2 (14.3%) of 14 patients on fondaparinux. There was no significant difference in the overall risk of bleeding among the regimens (Wald’s test P value .108). These results as well as individual regimen comparisons are presented in Table 4.
Table 4.
Bleeding Episodes During First Anticoagulant by Type of Initial Anticoagulation.
| Anticoagulant Regimen | Patients on Regimen | Bleed, # (%) | 3-Month Bleed Incidence, (%) | 6-Month Bleed Incidence, (%) | Incidence per 1000 Person-Months |
|---|---|---|---|---|---|
| Warfarin | 132 | 14 (10.6%) | 4 (3.0%) | 7 (5.3%) | 8.3 |
| Enoxaparin | 53 | 9 (17.0%) | 4 (7.5%) | 6 (11.3%) | 24.7 |
| Dalteparin | 37 | 10 (27.0%) | 2 (5.4%) | 3 (8.1%) | 28 |
| Fondaparinux | 14 | 2 (14.3%) | 0 (0.0%) | 0 (0.0%) | 9.9 |
| Fisher exact | 0.089 |
Patient Outcomes
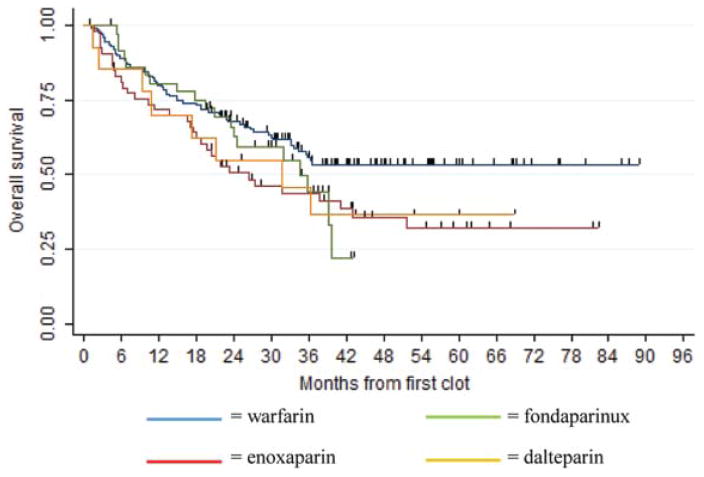
Median follow-up time for all patients was 43.3 months. Overall survival by anticoagulation type did not differ significantly between the regimens (log-rank P value .1041) and is shown in supplemental Figure 2. For warfarin, median OS was not reached, for enoxaparin it was 26.5 months, for dalteparin 34.5 months, and for fondaparinux 31.8 months. At 12 months, 80.2% of the patients initially treated with warfarin remained alive, compared to 71.7% of patients treated with enoxaparin, 80.8%with dalteparin, and 70.4%with fondaparinux. The most common cause of death in all patients was progressive malignancy. There were no fatalities related to bleeding.
DISCUSSION/CONCLUSIONS
We reviewed the rate of recurrent VTE in patients with malignancy referred to an AMS dedicated to patient with cancer at our institution. Patients treated with warfarin had a low rate of VTE recurrence of 5.3%, which compared favorably to 10.6% in patients treated with parenteral agents, with similar or even lower rates of bleeding. These results differ from those reported in several randomized controlled trials of warfarin versus a parenteral agent in patients with cancer including the largest study, the CLOT trial, in which there was a 17% recurrence rate for patients treated with warfarin compared to 9% in the dalteparin group at 6 months.11,13–15 Reanalysis of data from the CLOT trial, however, using competing risk techniques and Gray test found that the original Kaplan-Meier method overestimated risk of recurrent VTE in both treatment groups by about 30%. Those treated with LMWH still had a significantly lower risk of recurrent clot than those treated with a vitamin K antagonist, but the magnitude of effect was not as large.16,17
Our patient population differs in many ways from those enrolled in randomized trials of warfarin versus an LMWH for treatment of first VTE in patients with cancer. Both referral bias and selection bias are inherent characteristics of this population which cannot adequately be controlled for. The majority of patients were referred to our AMS for warfarin initiation and management either because their primary oncologist felt that warfarin was an acceptable anticoagulant or because of inability to use parenteral agents for reasons such as cost or intolerance of injections. The AMS staff assisted in obtaining LMWH at reduced cost if possible—especially for those patients receiving chemotherapy regimens that significantly interfered with warfarin metabolism—but were not often successful. Some patients treated with a parenteral agent may have been referred to the AMS for the close monitoring the service provided, as they were considered challenging due to perceived high risk of bleeding or recurrent thrombosis. These factors, as well as the retrospective nature of this study, are limitations of our findings.
Our patient population was at high risk of recurrent VTE events. Of the patients, 28.4% had a diagnosis of hematologic malignancy, which confers a significantly increased risk of primary VTE events compared to low-risk solid tumors; these patients have been excluded from many clinical trials of anticoagulation in patients with cancer due to concern for excess bleeding. Of the patients with solid tumors, 58% had metastatic disease. Despite increased VTE risks in our population, recurrence rates with warfarin are comparable to reported rates for VTE recurrence in general patient populations on warfarin therapy. A recurrence rate of 7% was seen in a prospective observational study of 15 000 patients.18
We have found that warfarin can be used successfully to treat VTE in patients with active cancer with acceptable rates of VTE recurrence and bleeding complications, especially in those who have no alternative anticoagulant choices. Our results are similar to a retrospective study in which the rate of recurrent VTE in 145 patients with breast cancer managed by a dedicated AMS was 4.1% with an average TTR of 53%.19 Warfarin management in patients with cancer is challenging and requires more frequent INR monitoring than in other patient populations, such as those with atrial fibrillation. Drug–drug interactions occur frequently with many chemotherapy regimens,20 but careful monitoring and dose adjustment by dedicated staff can mitigate significant variations in INR results, as evidenced by our center TTR of 59.5%. Although current ACCP, ASCO, and NCCN guidelines recommend the use of an LMWH for the first 3 to 6 months of treatment of first acute VTE in patients with malignancy,21–23 for those patients unable to use a parenteral agent, we have demonstrated that warfarin can be used to prevent recurrent VTE events.
Figure 2.
Kaplan-Meier survival estimates by anticoagulant regimen.
Acknowledgments
FUNDING:
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Statistical analyses were supported in part by the Dana-Farber/Harvard Cancer Center Core Grant 5P30 CA-006516.
Footnotes
The findings in this article were presented in poster format at the Thrombosis and Hemostasis Societies of North America meeting in Chicago, Illinois in 2014. ALM designed the study, collected the primary data, collaborated in data analysis, collaborated in data interpretation, wrote the first draft and contributed to revised drafts of the manuscript, and gave final approval before manuscript submission. FC collaborated in data analysis, contributed to revised drafts of the manuscript, and gave final approval before manuscript submission. DN supervised the data analysis, contributed to revised drafts of the manuscript, and gave final approval before manuscript submission. BR assisted in collection of the primary data and gave final approval before manuscript submission. JMC designed the study, supervised the data interpretation, contributed to revised drafts of the manuscript, and gave final approval before manuscript submission.
DECLARATION OF CONFLICTING INTERESTS:
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lillicrap D. Introduction to a series of reviews on cancer associated thrombotic disease. Blood. 2013;122(10):1687–1688. doi: 10.1182/blood-2013-05-499087. [DOI] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep venous thrombosis and pulmonary embolism: a population based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 4.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002;87(4):575–579. [PubMed] [Google Scholar]
- 5.Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 6.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848–4857. doi: 10.1200/JCO.2009.22.8197. [DOI] [PubMed] [Google Scholar]
- 8.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 9.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 11.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 12.Weingart SN, Cleary A, Scullion B, et al. Comparing clinicians’ use of an anticoagulation management service and usual care in ambulatory oncology. J Oncol Pharm Pract. 2013;19(3):237–245. doi: 10.1177/1078155212464892. [DOI] [PubMed] [Google Scholar]
- 13.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 14.Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12(4):389–396. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 15.Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Campigotto F, Neuberg D, Zwicker JI. Biased estimation of thrombosis rates in cancer studies using the method of Kaplan and Meier. J Thromb Haemost. 2012;10(7):1449–1451. doi: 10.1111/j.1538-7836.2012.04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parpia S, Julian JA, Thabane L, Lee AY, Rickles FR, Levine MN. Competing events in patients with malignant disease who are at risk for recurrent venous thromboembolism. Contemp Clin Trials. 2011;32(6):829–833. doi: 10.1016/j.cct.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Lobo JL, Jimenez D, Teresa Orue M, et al. Recurrent venous thromboembolism during coumarin therapy. Data from the computerized registry of patients with venous thromboembolism. Br J Haematol. 2007;138(3):400–403. doi: 10.1111/j.1365-2141.2007.06679.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones KL, Barnett C, Gauthier M, Boster B, Espirito JL, Michaud LB. Clinical outcomes of a pharmacist-managed anticoagulation service for breast cancer patients. J Oncol Pharm Pract. 2012;18(1):122–1227. doi: 10.1177/1078155210397775. [DOI] [PubMed] [Google Scholar]
- 20.Pangilinan JM, Pangilinan PH, Jr, Worden FP. Use of warfarin in the patient with cancer. J Support Oncol. 2007;5(3):131–136. [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network. [Accessed January 23, 2014];Venous Thromboembolic Disease. Web site http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
- 22.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):19S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]