Abstract
A crushed non-encapsulated CdTe thin-film solar cell was subjected to two standardized batch leaching tests (i.e., Toxicity Characteristic Leaching Procedure (TCLP) and California Waste Extraction Test (WET)) and to a continuous-flow column test to assess cadmium (Cd) and tellurium (Te) dissolution under conditions simulating the acidic- and the methanogenic phases of municipal solid waste landfills. Low levels of Cd and Te were solubilized in both batch leaching tests (<8.2% and <3.6% of added Cd and Te, respectively). On the other hand, over the course of 30 days, 73% of the Cd and 21% of the Te were released to the synthetic leachate of a continuous-flow column simulating the acidic landfill phase. The dissolved Cd concentration was 3.24-fold higher than the TCLP limit (1 mg L-1), and 650-fold higher than the maximum contaminant level established by the US-EPA for this metal in drinking water (0.005 mg L-1). In contrast, the release of Cd and Te to the effluent of the continuous-flow column simulating the methanogenic phase of a landfill was negligible. The remarkable difference in the leaching behavior of CdTe in the columns is related to different aqueous pH and redox conditions promoted by the microbial communities in the columns, and is in agreement with thermodynamic predictions.
Keywords: CdTe thin-film solar panel, landfill, leaching, cadmium, tellurium
1. Introduction
In recent years, solar photovoltaic (PV) technology has advanced due to a growing interest in renewable energy sources. While crystalline silicon has remained the dominant PV technology, thin-film solar panels have become increasingly popular [1]. The leading thin-film technology, cadmium telluride (CdTe), had a module production of 1.8 GWp in 2012, making it the second largest PV technology on the market [2]. Due to their efficiency and relatively low manufacturing energy requirements, CdTe solar cells had the shortest energy payback time among commercially relevant PV technologies [3].
As their usage has increased, there has also been increased concern over the safety of CdTe solar cells because of the toxic compounds they contain. Cadmium is recognized as a toxic substance by the United States Environmental Protection Agency (EPA), which set a maximum contaminant level (MCL) for cadmium (Cd) of 0.005 mgL-1 in drinking water. Tellurium (Te), while not regulated by the EPA, has also been shown to have the potential to cause kidney, heart, skin, lung, and gastrointestinal system damage in rats and in humans [4, 5]. Furthermore, two individuals that were mistakenly injected with sodium tellurite died after 6 h of exposure [4]. The toxicity of CdTe itself is poorly characterized, but several studies have shown that CdTe [6, 7] and CdTe quantum dots are cytotoxic to mammalian cells [8-10]. CdTe can cause severe pulmonary inflammation and fibrosis [6]. Zayed and Philippe (2009) [11] studied the acute toxicity of CdTe to rats via nasal and oral routes. The median lethal concentration of aerosolized CdTe in the nasal exposure test was 2.7 mg CdTe L-1 atmosphere (particles were 2-3 μm in diameter). The acute toxicity was also evaluated with oral gavage administration together with carboxymethylcellulose. In the oral route the highest body weight dose of 2g kg-1 was below the lethal concentration in a 14-d observation period.
Electronic waste is often disposed in municipal mixed solid waste (MSW) landfills [12, 13]. For example, in Australia, 84% (by weight) of electronic waste generated in 2008 was landfilled [14]. In the United States, electronic waste has been reported to account for 70% of the Cd and lead (Pb) present in landfills [15]. Disposal of electronic waste into landfills raises concerns about the potential release of toxic compounds into the environment. The US EPA uses the Toxicity Characteristics Leaching Procedure (TCLP) to characterize the potential of a solid waste to leach when disposed in a landfill and determine whether a waste material should be classified as hazardous according to its toxic characteristic [16]. If the waste fails the TCLP test, it must be disposed of in a hazardous waste landfill. The TCLP limit for cadmium is 1 mgL-1. While some TCLP leaching studies have reported concentrations of Cd lower than 1 mg L-1 [17], others have obtained Cd concentrations exceeding 9 mgL-1 for CdTe solar cells [18, 19].
Due to the presence of hazardous substances like Cd and lead (Pb) in PV technology, solar PV panels have been included in the European Union Waste Electrical and Electronic Equipment (WEEE) Directive [20] which is aimed at maximizing the collection, recycling and recovery of valuable and hazardous materials from electronic waste to optimize the use of natural resources, as well as, to prevent the entry of the toxicants into the environment. Despite the potential risks posed by PV technology, many other countries have not yet introduced regulations to prevent the disposal of CdTe solar cells in MSW landfills.
While some studies have assessed the chemical dissolution of Cd and Te from CdTe solar panels using different standardized tests simulating outdoor exposure and the landfill environment [17, 21-24], none have considered the potential impacts of the complex biogeochemical conditions commonly found in municipal MSW landfills on the mobility of these toxic contaminants. For instance, the pH of landfill leachates varies widely from highly acidic values typical of young landfills to circumneutral and slightly alkaline pH values characteristic of mature landfills [25, 26]. Such pH shifts could impact the dissolution of CdTe in the landfill leachate since both the corrosion of CdTe [27] and the aqueous solubility of Cd and Te are strongly dependent on pH [28, 29]. Municipal waste landfills are generally rich in anaerobic microbial activity. Anaerobic microorganisms are capable of reducing and precipitating tellurium [30-33], and such microbial transformations could potentially impact the dissolution of CdTe in the landfill environment. Microbial processes have previously been shown to promote the mobilization of some metals and metalloids in MSW landfills. For example, several studies have demonstrated the microbially-mediated mobilization and biotransformation of arsenic under simulated landfill conditions [34-36]. A preliminary generic risk assessment for decommissioned thin-film CdTe panels disposed in a MSW landfill has been performed using a U.S. EPA software model based on TCLP data that concluded that under current production of PV panels there should be no risk to humans [24, 37].
The purpose of this study is to investigate the potential environmental risk posed by the disposal of CdTe solar cells in municipal waste landfills. For this purpose, crushed samples of CdTe cells were placed in continuous-flow columns simulating the chemical, physical, and biological environments at two of the different stages in the lifetime of a landfill (i.e. the acidic phase and the methanogenic phase) [25]. The release of both Cd and Te was then monitored to determine the potential hazards of this disposal method.
2. Materials and methods
2.1. Panel characteristics
A thin-film CdTe solar panel was obtained from Abound Solar (Loveland, CO, USA) with dimensions of 60 by 120 cm, and 14 kg in mass. The CdTe thin-film layer was deposited between two sheets of glass, one of the sheets served as a glass substrate; while, the other was used as a back cover. The Abound solar panel used did not contain a laminate layer (e.g. ethylvinylacetate) to encapsulate the Cd-containing film. Such layer is found in some CdTe PV modules commercially available [38]. Four fragments of the panels were separated in order to determine the film to glass mass ratio of the panel as follows: the films were manually separated from their corresponding glass cases and, the masses of both fractions were obtained with an analytical balance (Mettler Toledo AB304-s; Columbus, OH, USA); finally, the film to glass mass ratio was estimated to be 0.0053 ± 0.00042 w/w.
2.2. Cadmium and tellurium content determination
Film fragments were digested using 15 mL of concentrated HF (45% wt.) and 3 mL of concentrated HNO3 (70% wt.) according to a modified EPA standard procedure [39]. Digested samples were then diluted in demineralized (DI) water and analyzed for Cd and Te. The samples were analyzed with an inductively coupled plasma–mass spectrometer (ICP-MS, Agilent model 7700x; Santa Clara, CA, USA) operated with He as collision gas to reduce Ar based interferences. The detection limits were 1.6 ng L-1 for Cd and 32.5 ng L-1 for Te. The panel was found to contain 7.31 ± 1.22 mg Cd and 7.68 ± 1.19 mg of Te per gram of film, respectively.
2.3. Panel preparation
The size of the panel was first reduced by breaking it with a hammer and the larger fragments were placed into a ball mill (Sepor, Wilmington, CA, USA). The milling process was carried out at 90 rpm. The CdTe-containing film is not brittle and was poorly fragmented during milling. Therefore, the film fragments were manually separated from the glass fragments and their size further reduced with scissors. The length of the longest side of the snipped film ranged between 3-8 mm and the thickness of the film was < 1 mm. The glass fragments were then sieved using a standard 3 1/2 mesh sieve (ASTM E11). The fragments that did not pass through were then sieved using a standard mesh no. 14 (ASTM E11), and the fragments that passed through were kept, resulting in glass fragments that ranged in size from 1.4–5.6 mm.
2.4. Microbial inoculum
A methanogenic anaerobic granular sludge obtained from a wastewater treatment plant at Mahou beer brewery (Guadalajara, Spain) was used to inoculate the columns. The inoculum contained 0.079 g of volatile suspended solids (VSS) g-1 wet wt. The maximum acetoclastic and hydrogenotrophic methanogenic activities of the sludge were 566 ± 64 mg methane as chemical oxygen demand (COD-CH4) g VSS−1 day−1 and 571 ± 26 mg COD-CH4 g VSS−1 day−1, respectively.
2.5. Columns set up and operational conditions
Two continuous upflow columns (V= 280 mL) were prepared and operated in parallel to model different stages of a MSW landfill (Fig. 1). Each column was packed with 1.5 g of snipped CdTe film and 300 g crushed glass, corresponding to the same mass ratio as was present in the panels. Each column contained 11.0 mg of Cd and 11.56 mg Te. Column 1 (acidic column) and column 2 (methanogenic column) were used to study the effect of the leachate produced during the acidic phase (young landfill) and in the initial methanogenic phase (mature landfill, circumneutral pH) of a municipal landfill, respectively, on the dissolution and mobility of the Cd and Te species present in the solar panel. Both columns were inoculated with 9.80±0.03 g sludge-VSS L-1. The sludge used to inoculate the acidic column was heat treated in a VWR convection oven (1370GM, Radnor, PA, USA) at 70°C for 60 min to pasteurize the methanogenic microbial communities and thereby prevent an increase of the pH of the synthetic leachate due to the volatile fatty acids(VFA) degradation.
Figure 1.
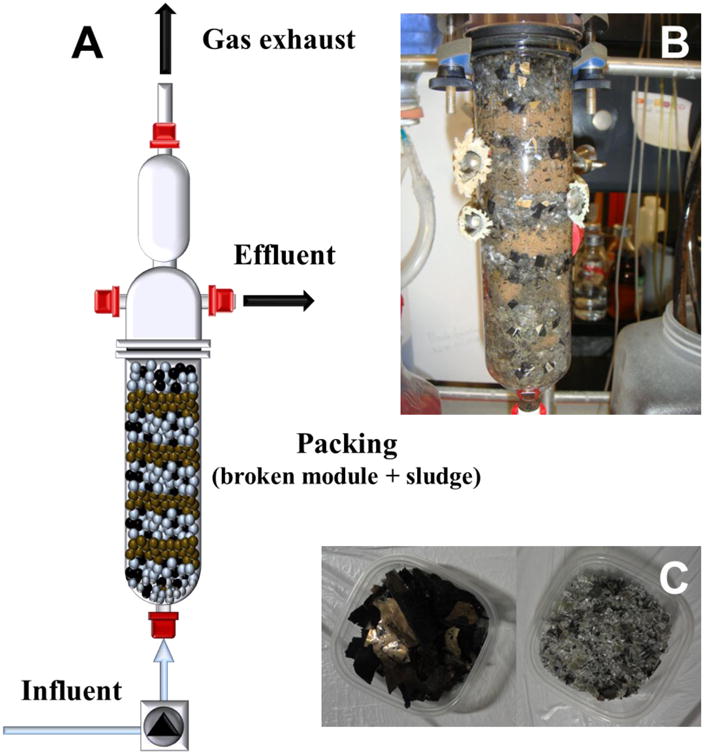
Schematic of the continuous columns used in this work (panel A). Image of the layered packing of the columns (panel B). Snipped CdTe film and crushed glass fractions of solar panels used in the column packing (panel C).
The columns were fed a synthetic leachate solution composed of a mixture of VFA (please refer to the Supplementary Data (SD) section) with a final pH of 4.67 ± 0.06. The pH of the influent of the acidic column was kept at 4.67 ± 0.06. The pH values for landfill leachate in this stage have been reported to range between 4.5 and 7.5 [25]. The pH of the influent of the methanogenic column was adjusted to 6.71 ± 0.19 using 10 M NaOH since microbial methanogenesis occurs only within a narrow pH range (circumneutral).
The acidic and methanogenic columns were operated at 25°C for 30 days at an average hydraulic retention time (HRT) of 27.7 ± 1.9 h and 28.8 ± 2.2 h, respectively.
2.6. Column sampling and monitoring
Throughout the testing period, samples were taken of the influent and effluent of both columns to determine the pH, as well as, the concentration of soluble Cd, Te, and VFA using the analytical methods described in the SD section. The CH4 content of the gas produced in the columns was measured by monitoring the volume of liquid displaced in an inverted bottle filled with NaOH (2%, v/v) to scrub CO2.
2.7. Chemical oxygen demand mass balance
The chemical oxygen demand (COD) concentration in the inlet and outlet streams of the columns was estimated by adding the COD of all species in these streams (Eq. 1 and 2). The mass balance for the components in the synthetic leachate, expressed as total COD, is represented in Eq. 3.
| (1) |
| (1) |
| (3) |
Where: infl = influent, effl = effluent, C2 = acetate, C3 = propionate, C4 = butyrate, C5 = valerate, C6 = caproate, [COD]cells = organic matter COD used to produce new cells, and [COD]methane = organic matter COD used to produce methane.
The last term in Eq. 3 ([COD] cells) was not considered in the COD balances since the cell growth yield associated with the anaerobic conversion of volatile fatty acids to methane is very low, typically less than 2-5% of the COD utilized[40, 41].
2.8. Standardized leaching tests
The CdTe solar panel was subjected to the standardized TCLP [16] and the California Waste Extraction Test (WET) [42] leaching tests using the snipped film and crushed glass previously prepared for the continuous columns set up, at the appropriate film/glass mass ratio of ∼ 0.005. These standardized tests are used to determine if a solid waste should be considered and handled as hazardous waste. If the waste fails the standardized leaching test it must be disposed of in a hazardous waste landfill instead of in a MSW landfill along with non-hazardous waste. The experimental conditions used in this work are described in the SD section.
2.9. Redox potential vs. pH (Pourbaix) diagrams
In this work, the W32-STABCAL software (Stability calculation for aqueous and non-aqueous systems, Montana Tech, Butte, MT, USA) and thermodynamic information published by the National Bureau of Standards NBS (NIST, U.S. Department of Commerce, USA) were used to plot potential-pH (Pourbaix) diagrams for CdTe. These diagrams showed the thermodynamically stable species of Cd and Te in complex aqueous systems under different redox potentials and pH conditions.
3. Results
3.1. Leaching of soluble Cd and Te species from the solar panel
Two continuous-flow columns packed with a mixture of CdTe thin-film and crushed glass of solar panels were run in parallel to investigate the impact of two simulated landfill conditions on the potential release of soluble Cd and Te from the CdTe semiconductor film into the environment. The columns were set up to simulate the acidic phase (acidic column) and, the initial period of the anaerobic methanogenic phase (methanogenic column) of a MSW landfill.
Figure 2A shows the concentrations of Cd and Te in the effluent from the columns. In the acidic column, the concentrations increased during the first ten days, reaching maximum levels of 3.23 mg L-1 for Cd and 1.10 mg L-1 for Te, respectively. At this point of time, 47.7% and 12.6% of the total Cd and Te contained in the solar panel had been released to the effluent, respectively. The concentration of Cd in the effluent was found to be above the threshold limit established in the TCLP for this metal for approximately 50% of the full operation time. The concentration of both Cd and Te in the effluent then decreased over the remainder of the experiment. Figure 3A shows the cumulative release of Cd and Te in the acidic column. Over the course of 30 days, 73% of the Cd and 21% of the Te were released.
Figure 2.
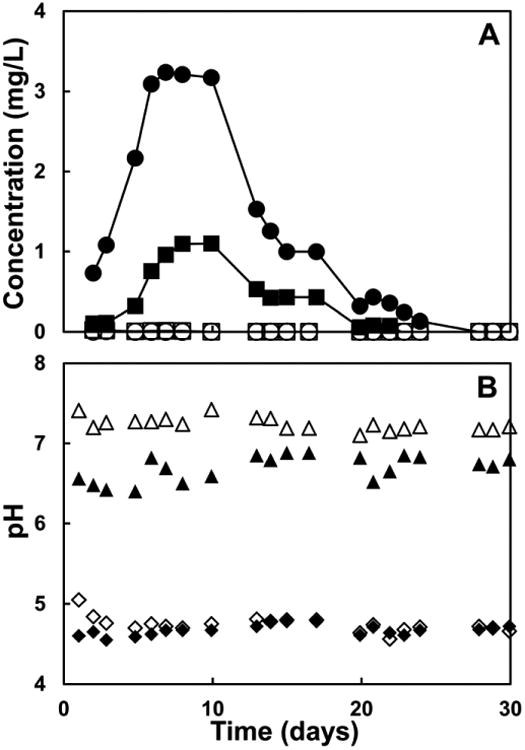
Release of soluble cadmium (Cd) and tellurium (Te) from a CdTe solar panel and pH of the effluent of continuous flow columns operated under simulated landfill conditions as a function of time. Panel A: Concentrations (in mg L-1) of Cd (●) and Te (■) in the effluent of the column simulating the conditions of a young, acidic landfill (pH 4.67 ± 0.06); and concentrations of Cd (○) and Te (●) in the effluent of the column simulating the conditions of a mature methanogenic landfill (pH 6.71 ± 0.19). Panel B: pH of influent (◆) and effluent (◊) of the column simulating an acidic landfill; pH of the influent (▲) and effluent (Δ) of the column simulating a methanogenic landfill.
Figure 3.
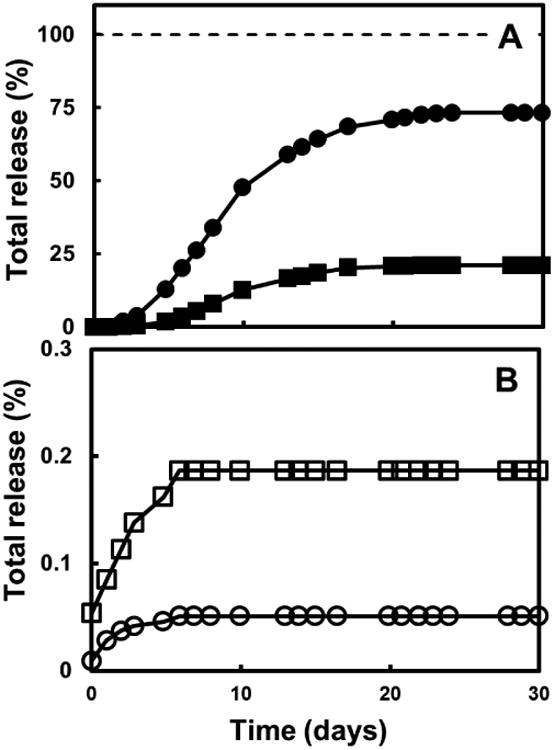
Time course of the cumulative release of soluble cadmium (●, ○) and tellurium (■, □) from the solar panel in the continuous columns (as % of the total initial metal concentration supplied as CdTe). Panel A: Column simulating the conditions of an acidic landfill. Panel B: Column simulating the conditions of a methanogenic landfill.
In the methanogenic column, only very low concentrations of Cd and Te were measured in the effluent during the first 8 days of the experiment, and afterwards these contaminants could no longer be detected in the effluent for the remainder of the experiment. The maximum levels of Cd and Te observed in the effluent were 0.01 mg Cd L-1 (on the third day of operation), and 0.02 mg Te L-1 (on the second day of operation), respectively. Only 0.03% and 0.05% of the total Cd and Te in the solar panel, respectively, had been released to the effluent when their maximum levels were observed. Figure 3B shows the cumulative release in the methanogenic column. In total, 0.05% of the Cd and 0.18% of the Te were released.
Figure 2B shows that the pH of the effluent of both columns remained stable and very close to the pH of the respective influents over the course of the experiment.
3.2. Chemical oxygen demand balance
The continuous columns used in this work were fed with a synthetic leachate solution composed of a VFA mixture with an initial concentration of approximately 1.5 g COD L-1. To monitor the activity of the microbial communities in the anaerobic sludge, the concentration of the synthetic leachate of the influent and effluent, as well as, the production of methane in both columns were measured during the full time of operation. Equations 2 and 3 were used to estimate the total COD in the influent and effluent of the columns, respectively.
Figure 4A shows the time course of the COD concentration of the synthetic leachate in the influent and effluent of the methanogenic column. The COD concentration decreased progressively from its initial value in the influent until day 20, when the microorganisms in the anaerobic sludge were able to completely degrade the VFA mixture. Figure 4B shows the daily production of methane in the methanogenic column, as well as, the maximum expected production of CH4 (0.367±0.036 g COD-CH4 day-1) based on the amount of COD added to the influent of the column. The daily methane production increased over time until it reached the maximum expected value at day 20 and remained stable until the last day of operation At that point of time, the mass balance in Eq. 3 can best be represented as Σ[COD]infl = [COD] methane.
Figure 4.
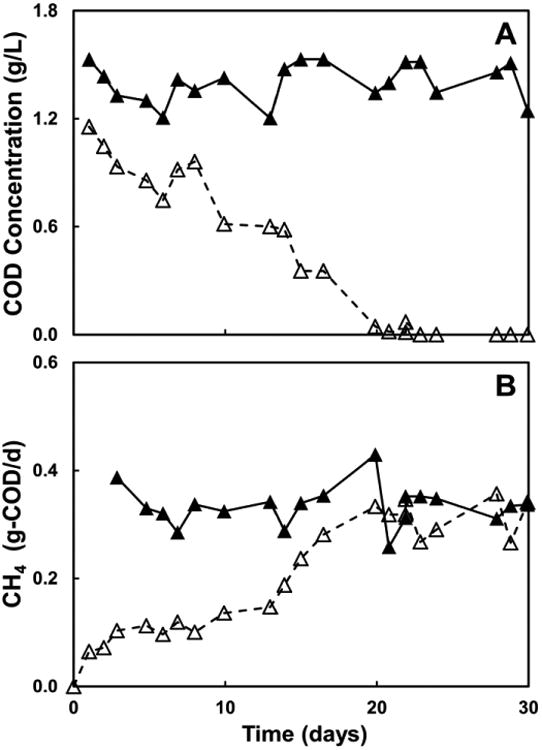
(Panel A) COD concentration in the influent (▲) and effluent (Δ) of the methanogenic column (pH 6.71± 0.19). (Panel B) Daily production of methane as a function of the operation time: (▲), maximum expected concentration based on the amount of COD added to the influent; (Δ), daily measured production.
In the case of the acidic column, no degradation of the VFA in the synthetic leachate or methane production was observed in the full time of operation as can be observed in Figure S1A (in supplementary data SD). This is consistent with the fact that the inoculum of the acidic column was subjected to heat treatment to inhibit methanogenic microorganisms. The low pH values of the column contents of the acidic column (pHeffluent = 4.74±0.10, Fig. 2B) were also below the optimal range for methanogenesis.
3.3. Standardized leaching test
In this study, fragments of the CdTe solar panel were subjected to the EPA TCLP test and to the California WET leaching test to assess the potential dissolution of the toxic compounds present in the semiconductor material.
Figure 5 shows the results for the TCLP and WET test performed with the CdTe solar panel. The concentration of soluble Cd determined in the TCLP and WET tests were 0.150±0.006 and 0.219±0.010 mg L-1, respectively. The Cd concentration was 6.7-fold lower than the threshold limit established in the TCLP test and 4.6-fold lower than the one for the WET test. The concentration of soluble Te in the final solutions produced in the TCLP and WET tests were 0.069±0.008 and 0.074±0.011 mg L-1, respectively.
Figure 5.
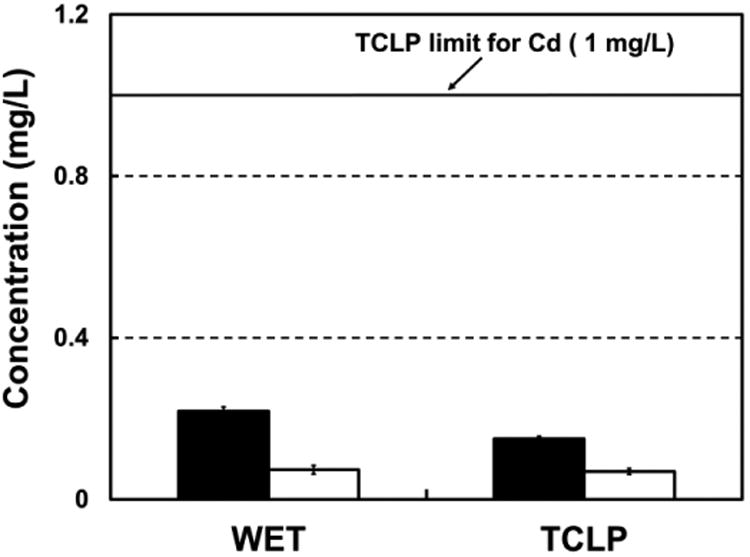
Concentrations of soluble Cd (filled columns) and soluble Te (blank columns) released from the CdTe solar panel in the US-EPA Toxicity Characteristics Leaching Procedure (TCLP) and the California Waste Extraction Test (WET) leaching tests.
4. Discussion
4.1. Main findings
4.1.1 Leaching of Cd and Te
The potential release of the toxic compounds from a CdTe thin-film solar panel under conditions simulating those prevailing in young- and a mature MSW landfills was assessed in this work. The most important effect was observed in the acidic column in which a remarkable release of Cd was observed (ca. 73% of the Cd supplied as CdTe). The maximum concentration of Cd in the leachate was 3.24 mg L-1, which is 3-fold higher that the TCLP limit for Cd, and 650-fold higher than the U.S. federal MCL in drinking water (0.005 mg L-1). Comparison of Cd levels in the leachate with MCL values provides information on the required attenuation to ensure that MLC levels are not exceeded in drinking water resources impacted by landfill leachate. The maximum concentration of dissolved Te measured in this leachate was 1.1 mg L-1. In contrast, the release of dissolved Cd and Te species was negligible in the methanogenic column. To the best of our knowledge, this study represents the first attempt to comparatively assess the leaching behavior of a CdTe solar panel by considering both simulated acidic and methanogenic landfill conditions in a continuous flow fashion.
Whereas the maximum flux of dissolved Cd detected in the acidic column was 0.246 mg Cd cm-2-cross section of the column hr-1, it is important to mention that the solar panel used in this study contained low levels of Cd and Te compared to the amount reported for other CdTe panels in several other studies. The PV cell used in this work contained 0.843±0.187 g Cd, and 0.891±0.211 g Te per m2 of intact panel representing a mass percentage of 0.004 ± 0.001% of each in the solar panel. Other works have reported contents of Cd and Te which are 7.4- fold to 74.6-fold higher than those used in this study [21, 22, 43]. The amount of Cd and Te in solar panels has been decreasing over time in an attempt to reduce production costs and comply with environmental restrictions [44, 45]. The large variability in Cd and Te content suggests that higher concentrations of soluble Cd and Te could be released if older CdTe solar cells are disposed in MSW landfills after they reach the end of their useful service life (25-35 years).
The findings of the present work correlate well with the results reported previously in chemical dissolution that used CdTe powder. Leaching tests conducted by Zeng et al. [27] demonstrated that CdTe has a high leaching potential, especially under acidic and aerobic conditions. Their study demonstrated that the concentrations of Cd released from CdTe powder when subjected to the TCLP and WET leaching tests were about 1500 and 260 times higher than the regulatory limit (1 mg L-1). The concentrations of soluble Cd determined in the present study are markedly lower than those obtained in the leaching studies performed with pure CdTe because CdTe solar cells contain a very large mass fraction of inert glass and the CdTe is present in a film.
The potential dissolution and mobility of Cd and Te from CdTe solar panels due to physico-chemical factors have been previously assessed using standardized batch leaching tests, and in one case, a column set up was used assessing leaching by rain. The TCLP data determined previously for CdTe solar panels show a large variability and soluble Cd values ranging from less than 0.15 to over 9.5 mg Cd L-1 (i.e., almost 10-fold higher than the TCLP threshold) have been reported [18, 19, 23, 46]. Two standardized leaching tests were conducted according to the European Union landfill directive and the U.S. TCLP on CdTe modules manufactured by the British Petroleum Company (BP) [46]. The concentrations of Cd in the produced leachates were lower than the thresholds limits established in the corresponding protocols. In a different study, a standardized batch leaching tests was performed according to the Norwegian Standard-European Norm Characterization of waste. This test uses deionized water as the leaching solution. The concentration of Cd in final leachate was found to be below the threshold limit established for an MSW landfill (≤1 mg Cd kg-1 dw.) [22]. In a study performed to investigate the impact of rain conditions on the mobility and emissions of soluble Cd and Te species in the environment, the concentrations of dissolved Cd and Te released after one week of exposure were 1.0 and 0.3 mg L-1, respectively[21].
4.1.2 Microbial communities and redox conditions
The degradation of solid waste in MSW landfills occurs through a series of complex chemical and biological reactions [25]. During the acid phase of a landfill, cellulose and hemicellulose are hydrolyzed by bacteria to form monosaccharides, which are fermented by fermentative bacteria to volatile fatty acids. In this stage, the lowest pH of the leachate was reported to be 4.5 [25]. The synthetic leachate used in this work was constituted by a mixture of carboxylic acids representative of those formed by fermentative bacteria during the acidic phase. The initial methanogenic phase starts when the pH of the leachate increases and a minimal growth of methanogenic microorganisms begins. Methanogenic microorganisms are highly sensitive to pH, and methanogenesis occurs only within a narrow pH range (circumneutral). In the methanogenic phase, acetogenic bacteria convert carboxylic acids to acetate, H2 and CO2 which in turn are converted to methane by methanogens.
In the methanogenic column, the VFAs in the synthetic leachate were assumed to be converted via acetate, H2 and CO2 by acetogenic bacteria in the sludge. Since the pH of the influent of this column (6.71±0.19) was favorable for the growth of methanogenic microorganisms, methane was progressively formed until a stable production of methane was observed. Due to the expected production of H2 by the acetogenic bacteria, and to the fact that methanogens are highly sensitive to oxygen, it is acceptable to assume that strong reducing conditions prevailed in this column.
4.2. Mechanisms of CdTe dissolution
The different leaching behaviors observed for CdTe in the two columns operated in the present study might be explained by the different pH levels and by differences in the redox conditions promoted by the microbial communities present in the methanogenic inoculum. Figure 6 shows the Pourbaix diagram obtained for the CdTe-H2O system. The diagram was generated using the highest concentration of each element observed in the effluent of the acidic column. The dashed lines represent the stability region of water and can be defined by the following equations:
Figure 6.
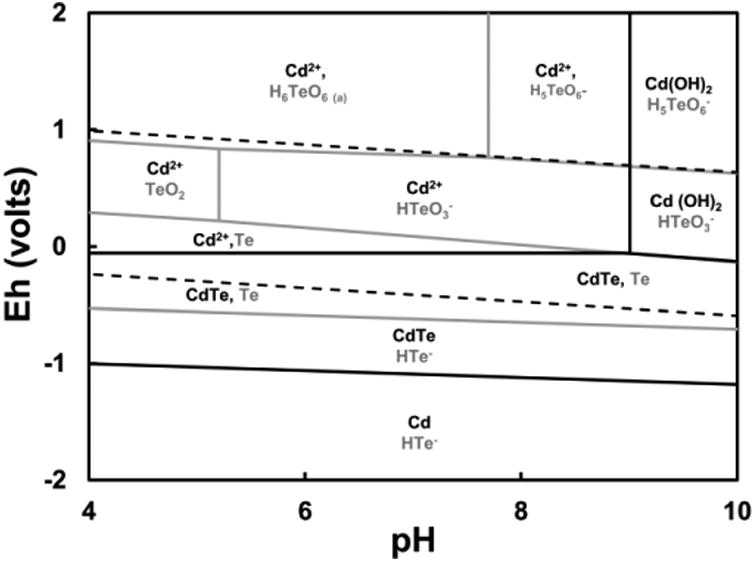
Pourbaix diagram for the CdTe – H2O system at 25°C. Pourbaix diagrams were constructed at activities of 2.9 × 10-2 mM Cd, and 8.6 × 10-3 mM Te.
| (4) |
| (5) |
In the region above the upper dashed line (Eq. 4) water releases oxygen. The region below the dashed lower line (Eq. 5) corresponds to the conditions at which water becomes unstable and releases hydrogen.
As mentioned before, there was no substantial evidence of microbial activity in the acidic column based on the lack of VFA consumption and methane production. The resazurin redox indicator dye in the column effluent was pink indicating microaerophilic conditions that may have resulted from the lack of microbial consumption of traces of dissolved oxygen. Under these conditions, the presence of an important fraction of soluble Cd and Te would be expected based on thermodynamic considerations, which is in agreement with the extensive dissolution of CdTe observed in the acidic column. According to the Pourbaix diagram, the redox potential for the lower water stability is -0.276 V and the one for the upper limit is 0.954 V. The thermodynamically stable species expected for Te are elemental Te, HTeO3- (tellurite), and H6TeO6 (tellurate). The stable species for Cd are CdTe, and soluble Cd2+. The results are in agreement with our previous study investigating the leaching behavior of CdTe under different pH and redox conditions. Those results confirmed a marked enhancement in the dissolution of CdTe powder both at acidic pH levels and under aerobic conditions [27].
Due to the methanogenic microbial activity, highly reducing conditions are expected in the methanogenic column. CdTe is thermodynamically stable in aqueous solution under those conditions and no soluble Cd or Te would be expected in the system. According to the Pourbaix diagram, the redox potential for the lower water stability is -0.396 V, and the one for the upper line is 0.834 V. The thermodynamically stable species for Te are elemental Te and CdTe, and for Cd, CdTe is the only stable species. The absence of dissolved Cd in the circumneutral leachate could also be due to some extent to the decreased solubility of Cd2+ with increasing pH.
4.3. Implications
The evidence found in this work indicates that the standardized TCLP and WET leaching tests might underestimate the leaching of Cd and Te from disposing decommissioned CdTe solar panels in landfills. Although some previous works have stated that CdTe is an insoluble form of Cd and that CdTe is expected to have low bioavailability in the environment [47], the results obtained in the present study as well as another related investigations [27] indicate that a high fraction of the Cd and Te in CdTe panels could be potentially released if non-encapsulated CdTe solar panels are discarded in municipal landfills. Leaching of Cd and Te is expected to occur mainly during the acidic phase of a landfill in which low pH values are dominant.
The leaching results reported in the current study were obtained in accelerated column tests simulating the conditions in the acidogenic and methanogenic phases of a MSW landfill. The actual Cd concentrations in a given landfill would depend on the amount of PV panels disposed, panel design, panel fragment size, climatic conditions, landfill management and design, etc. The risk that leachate impact groundwater is minimized in modern MSW landfills that are designed with daily cover of the waste, storm water management, use of liners at the bottom of the landfill, and a leachate collection system [48]. Releases into groundwater are, thus, particularly a problem in landfills that were not originally designed to prevent migration of the leachate (unlined landfills). Elevated concentrations of metals and metalloids have been measured in samples of groundwater collected in locations close to landfills receiving electronic waste [49]. Furthermore, release of these highly toxic compounds into the landfill, could impact the microbial communities degrading organic waste and, thereby, the waste stabilization process. Our findings from a previous work indicated the methanogenic activity of anaerobic sludge are highly inhibited by the presence of Cd and Te soluble species [50].
The significant leaching of toxic Cd demonstrated in the present study under simulated, acidic landfill conditions indicates the need for further investigation of the leaching potential of decommissioned CdTe PV panels, and suggests the need for measures to minimize their disposal in MSW landfills. In this sense it is important to point out that, as previously mentioned, the European Union has recently adopted the WEEE Directive that aims to achieve a high degree of separation of PV panels and other electronic waste in order to minimize their disposal in MSW landfills, reduce the entry of toxicants into the environment, and ensure recycling and recovery of valuable and hazardous materials from these waste resources [20]. The U.S. Solar Energy Industries Association (SEIA) did also launch a solar PV recycling program in late 2016 to improve the environmental sustainability of the U.S. solar industry [51].
Conclusions
The biogeochemical conditions prevailing during the different stages of a landfill potentially affect the release of the toxic soluble compounds from decommissioned non-encapsulated CdTe PV cells. During the acidic phase of a young landfill, the highest release of Cd and Te will be expected as a result of the low pH prevailing in the landfill environment. In contrast, during the methanogenic phase of a mature landfill, low corrosion of CdTe caused by the highly reducing conditions in the landfill environment only allows for marginal release of soluble Cd and Te species. Given the high toxicity of Cd, these results suggest the need for measures to minimize the disposal of decommissioned CdTe PV panels in MSW landfills.
Supplementary Material
Acknowledgments
We are grateful to M.K. Amistadi from the Arizona Laboratory for Emerging Contaminants (ALEC) for the ICP-MS analysis. This work was funded in part by a grant of the National Institute of Environment and Health Sciences-supported Superfund Research Program (P42 ES04940). ARR was partly funded by CONACYT (171108) and PROMEP (UASLP-236).
References
- 1.Fraunhofer Institute for Solar Energy Systems; Germany: 2016. [last accessed: 03/04/2017]. Fraunhofer-ISE, Photovoltaics Report. https://www.ise.fraunhofer.de/content/dam/ise/de/documents/publications/studies/Photovoltaics-Report.pdf. [Google Scholar]
- 2.Kranz L, Buecheler S, Tiwari AN. Technological status of CdTe photovoltaics. Sol Energ Mat Sol C. 2013;119:278–280. [Google Scholar]
- 3.Leccisi E, Raugei M, Fthenakis V. The energy and environmental performance of ground-mounted photovoltaic systems-A timely update. Energies. 2016;9:13. [Google Scholar]
- 4.Taylor A. Biochemistry of tellurium. Biol Trace Elem Res. 1996;55:231–239. doi: 10.1007/BF02785282. [DOI] [PubMed] [Google Scholar]
- 5.Vij P, Hardej D. Evaluation of tellurium toxicity in transformed and non-transformed human colon cells. Environ Toxicol Pharmacol. 2012;34:768–782. doi: 10.1016/j.etap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Morgan DL, Shines CJ, Jeter SP, Blazka ME, Elwell MR, Wilson RE, Ward SM, Price HC, Moskowitz PD. Comparative pulmonary absorption, distribution, and toxicity of copper gallium diselenide, copper indium diselenide, and cadmium telluride in Sprague-Dawley rats. Toxicol Appl Pharmacol. 1997;147:399–410. doi: 10.1006/taap.1997.8267. [DOI] [PubMed] [Google Scholar]
- 7.Fthenakis VM, Morris SC, Moskowitz PD, Morgan DL. Toxicity of cadmium telluride, copper indium diselenide, and copper gallium diselenide. Prog Photovoltaics. 1999;7:489–497. [Google Scholar]
- 8.Liu L, Zhang J, Su X, Mason RP. In vitro and in vivo assessment of CdTe and CdHgTe toxicity and clearance. J Biomed Nanotechnol. 2008;4:524–528. doi: 10.1166/jbn.2008.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KC, Rippstein P, Tayabali AF, Willmore WG. Mitochondrial toxicity of cadmium telluride quantum dot nanoparticles in mammalian hepatocytes. Toxicol Sci. 2015;146:31–42. doi: 10.1093/toxsci/kfv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Hu Y, Tang M, Kong L, Ying J, Wu T, Xue Y, Pu Y. Liver toxicity of cadmium telluride quantum dots (CdTe QDs) due to oxidative stress in vitro and in vivo. Int J Mol Sci. 2015;16:23279–23299. doi: 10.3390/ijms161023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zayed J, Philippe S. Acute oral and inhalation toxicities in rats with cadmium telluride. Int J Toxicol. 2009;28:259–265. doi: 10.1177/1091581809337630. [DOI] [PubMed] [Google Scholar]
- 12.US EPA. United States Environmental Protection Agency; USA: 2011. [last accessed: 11/18/2016]. Electronic waste management in the United States through 2009. http://kuusakoski.us/wp-content/uploads/2013/11/summarybaselinereport2011.pdf. [Google Scholar]
- 13.Baldé CP, Wang F, Kuehr R, Huisman J. United Nations University, IAS – SCYCLE; Bonn, Germany: 2015. [last accessed: 11/18/2016]. The global e-waste monitor – 2014. https://i.unu.edu/media/unu.edu/news/52624/UNU-1stGlobal-E-Waste-Monitor-2014-small.pd. [Google Scholar]
- 14.EPHC. Environment Protection and Heritage Council (EPHC); Australia: 2009. [last accessed: 03/04/2017]. Decision regulatory impact statement: televisions and computers. http://www.nepc.gov.au/system/files/resources/0c513e54-d968-ac04-758b-3b7613af0d07/files/ps-tv-comp-decision-ris-televisions-and-computers-200911-0.pdf. [Google Scholar]
- 15.Widmer R, Oswald-Krapf H, Sinha-Khetriwal D, Schnellmann M, Boni H. Global perspectives on e-waste. Environ Impact Asses. 2005;25:436–458. [Google Scholar]
- 16.US EPA. Toxicity Characteristics Leaching Procedure. United States Environmental Protection Agency; USA: 1992. Method 1311. [Google Scholar]
- 17.Sinha P, Wade A. Assessment of leaching tests for evaluating potential environmental impacts of PV module field breakage. IEEE J Photovoltaics. 2015;5(6):1710–1714. [Google Scholar]
- 18.Cunningham D. Discussion about TCLP protocols in: Report on the workshop photovoltaics and the environment 1998, Brookhaven National Laboratory, Uptown, NY, USA, 1999 [Google Scholar]
- 19.Moskowitz PD, Fthenakis VM. Environmental, health and safety issues associated with the manufacture and use of II-VI-photovoltaic voltaic devices. Sol Cells. 1991;30:89–99. [Google Scholar]
- 20.The European Parliament and the Council of the European Union. Directive 2012/19/EU of the European Parliament and of the council of 4 July 2012 on waste electrical and electronic equipment (WEEE) (recast) Official Journal of the European Union. 2012;197:38–71. [Google Scholar]
- 21.Steinberger H. Health, safety and environmental risks from the operation of CdTe and CIS thin-film modules. Prog Photovoltaics. 1998;6:99–103. [Google Scholar]
- 22.Okkenhaug G. Norway: 2010. [last accessed: 03/04/2017]. Environmental risks regarding the use and end-of-life disposal of CdTe PV modules. https://www.dtsc.ca.gov/LawsRegsPolicies/upload/Norwegian-Geotechnical-Institute-Study.pdf. [Google Scholar]
- 23.Moskowitz PD, Steinberger H, Thumm W. Health and environmental hazards of CdTe photovoltaic module production, use and decommissioning, 1994; IEEE First World Conference on Photovoltaic Energy Conversion/Conference Record of the Twenty Fourth IEEE Photovoltaic Specialists Conference-1994, pp; pp. 115–118. [Google Scholar]
- 24.Sinha P, Trumbull VL, Kaczmar SW, Johnson KA. Evaluation of potential health and environmental impacts from end-of-life disposal of photovoltaics, in: M.A. Gill (Ed.) Photovoltaics: Synthesis, Applications and Emerging Technologies, NOVA Science Publishers, New York, 2014, pp. :37–52. [Google Scholar]
- 25.Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH. Present and long-term composition of MSW landfill leachate: A review. Crit Rev Env Sci Tec. 2002;32:297–336. [Google Scholar]
- 26.Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ, Heron C. Biogeochemistry of landfill leachate plumes. Appl Geochem. 2001;16:659–718. [Google Scholar]
- 27.Zeng C, Ramos-Ruiz A, Field JA, Sierra-Alvarez R. Cadmium telluride (CdTe) and cadmium selenide (CdSe) leaching behavior and surface chemistry in response to pH and O2. J Environ Manage. 2015;154:78–85. doi: 10.1016/j.jenvman.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Bouroushian M. Electrochemistry of metal chalcogenides, Springer-Verlag, Berlin, 2010 [Google Scholar]
- 29.Benjamin MM. Water Chemistry, Waveland Press, Inc, Long Grove, IL., 2002 [Google Scholar]
- 30.Baesman SM, Stolz JF, Kulp TR, Oremland RS. Enrichment and isolation of Bacillus beveridgei sp nov., a facultative anaerobic haloalkaliphile from Mono Lake, California, that respires oxyanions of tellurium, selenium, and arsenic. Extremophiles. 2009;13:695–705. doi: 10.1007/s00792-009-0257-z. [DOI] [PubMed] [Google Scholar]
- 31.Baesman SA, Bullen TD, Dewald J, Zhang D, Curran S, Islam FS, Beveridge TJ, Oremland RS. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl Environ Microb. 2007;73:2135–2143. doi: 10.1128/AEM.02558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basnayake RST, Bius JH, Akpolat OM, Chasteen TG. Production of dimethyl telluride and elemental tellurium by bacteria amended with tellurite or tellurate. Appl Organomet Chem. 2001;15:499–510. [Google Scholar]
- 33.Ramos-Ruiz A, Field JA, Wilkening JV, Sierra-Alvarez R. Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environ Sci Technol. 2016;50:1492–1500. doi: 10.1021/acs.est.5b04074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortinas I, Sierra-Alvarez R, Field JA. Biologically mediated mobilization of arsenic from granular ferric hydroxide in anaerobic columns fed landfill leachate. Biotechnol Bioeng. 2008;101:1205–1213. doi: 10.1002/bit.22021. [DOI] [PubMed] [Google Scholar]
- 35.Sierra-Alvarez R, Field JA, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. Anaerobic microbial mobilization and biotransformation of arsenate adsorbed onto activated alumina. Water Res. 2005;39:199–209. doi: 10.1016/j.watres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A, Mukiibi M, Saez AE, Ela WP. Leaching of arsenic from granular ferric hydroxide residuals under mature landfill conditions. Environ Sci Technol. 2006;40:6070–6075. doi: 10.1021/es060561b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cyrs WD, Avens HJ, Capshaw ZA, Kingsbury RA, Sahmel J, Tvermoes BE. Landfill waste and recycling: Use of a screening-level risk assessment tool for end-of-life cadmium telluride (CdTe) thin-film photovoltaic (PV) panels. Ener Policy. 2014;68:524–533. [Google Scholar]
- 38.Sinha P. Life cycle materials and water management for CdTe photovoltaics. Sol Energ Mat Sol C. 2013;119:271–275. [Google Scholar]
- 39.US EPA. Microwave assisted acid digestion of siliceous and organically based matrices. United States Environmental Protection Agency; USA: 1996. Method 3052. [Google Scholar]
- 40.Eddy M. Wastewater engineering, treatment and reuse, McGraw-Hill, New York, 2003 [Google Scholar]
- 41.Roden EE, Jin QS. Thermodynamics of microbial growth coupled to metabolism of glucose, ethanol, short-chain organic acids, and hydrogen. Appl Environ Microb. 2011;77:1907–1909. doi: 10.1128/AEM.02425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CCR. Waste Extraction Test (WET) Procedures; Title 22. Division 4.5, Chapter 11, Article 5, Appendix II., California Code of Regulation. 1991b [Google Scholar]
- 43.Fthenakis VM, Wang W. Extraction and separation of Cd and Te from cadmium telluride photovoltaic manufacturing scrap. Prog Photovoltaics. 2006;14:363–371. [Google Scholar]
- 44.Fthenakis VM. Sustainability of photovoltaics: The case for thin-film solar cells. Renew Sust Energ Rev. 2009;13:2746–2750. [Google Scholar]
- 45.Zweibel K. Issues in thin film PV manufacturing cost reduction. Sol Energ Mat Sol C. 1999;59:1–18. [Google Scholar]
- 46.Baumann AE, Hynes KM, Hill R. An investigation of cadmium telluride thin-film PV modules by impact pathway analysis. Renew Energ. 1995;6:593–599. [Google Scholar]
- 47.Patterson MH, Turner AK, Sadeghi M. Health, safety and environmental aspects of the use of cadmium compounds in thin-film pv modules. Sol Energ Mat Sol C. 1994;35:305–310. [Google Scholar]
- 48.US-EPA. United States Environmental Protection Agency; USA: 2017. [last accessed: 03/04/2017]. Municipal Solid Waste Landfill. https://www.epa.gov/landfills/municipal-solid-waste-landfills. [Google Scholar]
- 49.Kiddee P, Naidu R, Wong MH, Hearn L, Muller JF. Field investigation of the quality of fresh and aged leachates from selected landfills receiving e-waste in an arid climate. Waste Manage. 2014;34:2292–2304. doi: 10.1016/j.wasman.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Ramos-Ruiz A, Zeng C, Sierra-Alvarez R, Teixeira LH, Field JA. Microbial toxicity of ionic species leached from II-VI semiconductor materials, cadmium telluride (CdTe) and cadmium selenide (CdSe) Chemosphere. 2016;162:131–138. doi: 10.1016/j.chemosphere.2016.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.SEIA. Solar Energy Industries Association; USA: [last accessed: 03/04/2017]. SEIA National PV recycling program. http://www.seia.org/seia-national-pv-recycling-program. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


