Table 2.
Preparation of deuterodifluoromethyl ketones 27–35.
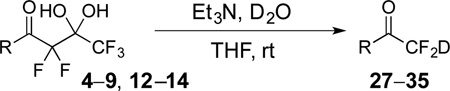 | ||||
|---|---|---|---|---|
| Entry | Substrate | Major Product | Yielda | % Db |
| 1 | 4 | 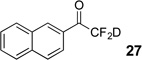 |
89% | 98% |
| 2 | 5 | 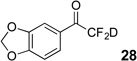 |
86% | 98% |
| 3 | 6 | 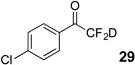 |
47% | 96% |
| 4 | 7 | 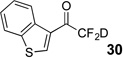 |
89% | 97% |
| 5 | 8 |  |
90% | 98% |
| 6 | 9 | 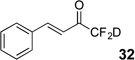 |
26%c | 99% |
| 7 | 12 | 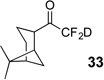 |
90% | 99% |
| 8 | 13 | 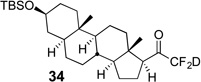 |
81% | 99% |
| 9 | 14 | 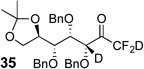 |
76% | 98% (d.r. = 4:3) |
Isolated yields.
Percent deuterium incorporation were determined by 19F NMR, see Supporting Information for details.
The major product was the dimer and it was isolated in 69% yield, see Supporting Information for details.
