Table 4.
Preparation of difluoromethyl sulfones 47–50 and deuterodifluoromethyl sulfones 51–54.
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Conditions | Major Product | Yielda | % Db |
| 1 | 43 | Et3N, H2O | 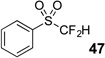 |
87% | na |
| 2 | 44 | Et3N, H2O | 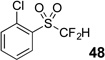 |
quant. | na |
| 3 | 45 | Et3N, H2O | 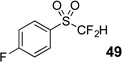 |
89% | na |
| 4 | 46 | Et3N, H2O | 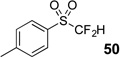 |
85% | na |
| 5 | 43 | Et3N, D2O | 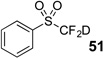 |
74% | 97% |
| 6 | 44 | Et3N, D2O |  |
96% | 96% |
| 7 | 45 | Et3N, D2O | 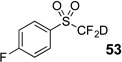 |
80% | 98% |
| 8 | 46 | Et3N, D2O | 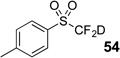 |
91% | 98% |
Isolated yields.
Percent deuterium incorporation were determined by 19F NMR, see Supporting Information for details.
