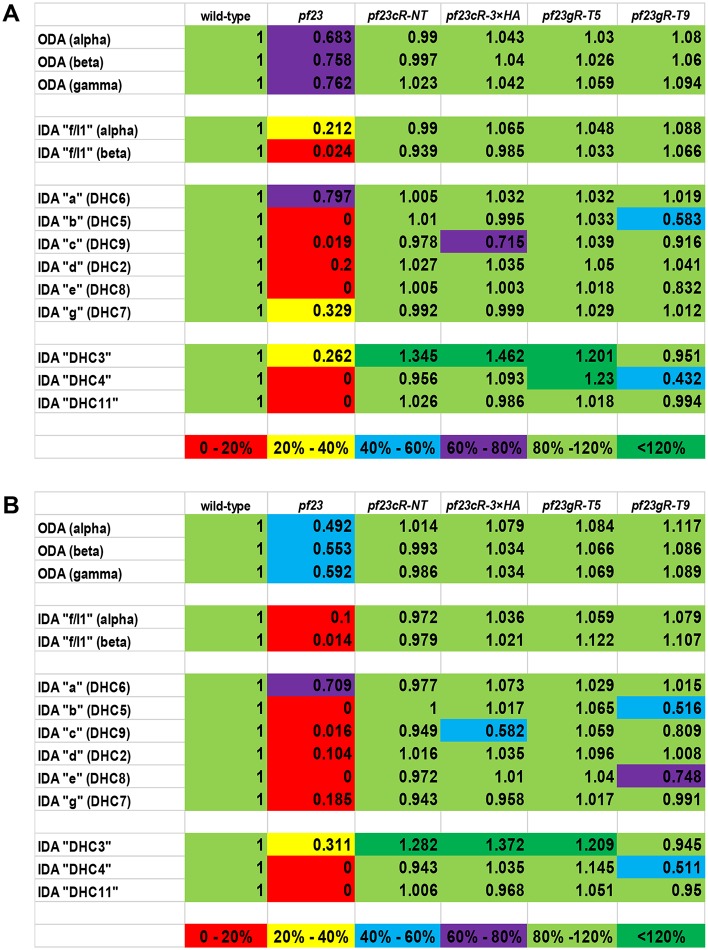
Fig 3. Spectral counting reveals failure of ciliary dynein assembly in pf23 axonemes.
(A, B) Ciliary dynein-containing regions of axonemal samples in the SDS-PAGE gels were cut from the gel, and the amount of each species of ciliary dynein estimated by spectral counting. The strains used were wild-type, pf23, pf23cR-NT, pf23cR-3×HA, pf23gR-T5 and pf23gR-T9, and results were normalized using the wild-type spectral numbers. In (A), a summary of dynein heavy chain peptide fractions (unique peptides) is shown that verifies the reliability of the spectral counting analyses. In (B), a summary of unweighted peptide analyses used to semi-quantitatively estimate the amount of ciliary dynein heavy chains is shown. The average of two independent experiments is presented in the figures. The colors are an indication of the percentage of wild-type level of each dynein heavy chain: red 0–20%, yellow 20–40%, blue 40–60%, purple 60–80%, light green 80–120%, dark green < 120%, respectively. The pf23 axonemes have greatly reduced amounts of major IDAs (“b”, “c”, “d”, “e”, “f/I1” and “g”), all below 20% of the wild-type level. In contrast, 70% of IDA “a” species is found in pf23 axonemes. The minor IDAs, “DHC3”, “DHC4” and “DHC11”, are also greatly reduced in pf23 axonemes. The ODA heavy chains are reduced about 50% compared to wild-type axonemes. In pf23 rescued strains, most of ciliary dyneins are recovered, but major IDAs “b”, “e” and minor IDA “DHC4” remain reduced in pf23gR-T9, which expresses a short DYX1C1 fragment lacking the wild-type C-terminus.