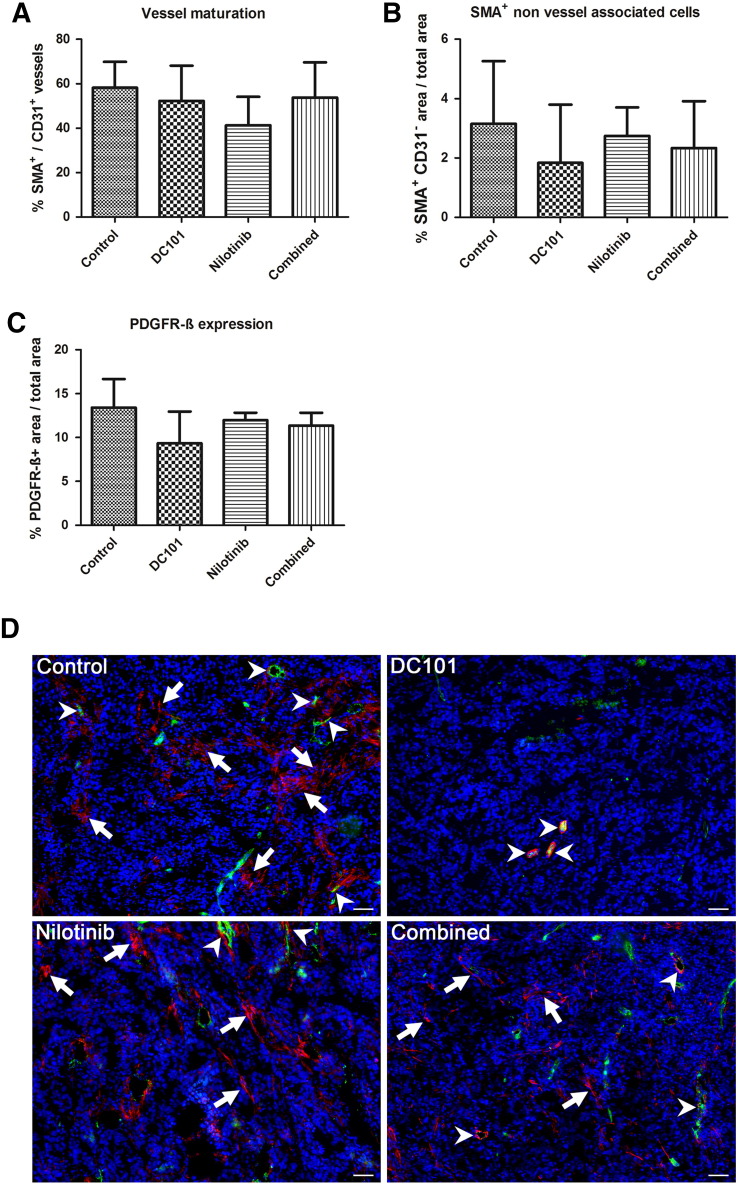
Figure 5.
Lower vessel maturation in nilotinib-treated tumors and treatment effects on myofibroblasts. (A) Quantification reveals that the ratio of α-SMA–positive vessels per total vessel fraction is lowest in nilotinib-treated tumors showing an impaired vessel maturation by PDGFR inhibition. (B) The fraction of non–vessel-associated α-SMA–positive cells (myofibroblasts) is markedly reduced in DC101-treated tumors, whereas in tumors of the nilotinib group, the amount of non–vessel-associated α-SMA–positive cells is only slightly lower than in the controls. (C) Quantification of the PDGFR-β–positive area fraction reveals a similar trend as for non–vessel-associated α-SMA, showing the lowest value for the DC101 treatment group, whereas the mean value for nilotinib-treated tumors is almost as high as in the controls. Data are presented as mean values ± standard deviation. (D) Representative immunofluorescent staining for α-SMA (red) and CD31 (green) in tumors of the control, DC101, nilotinib, and combined treatment groups at day 14. Counterstaining of nuclei in blue; arrows show non–vessel-associated α-SMA–positive cells, and arrowheads represent α-SMA–positive mature vessels. Scale bar: 50 μm.