| Problem | Typical cause | Suggested solutions |
| The impeller stops rotating during alginate or acid oil addition | The magnetic field is insufficient for adequate mixing or the impeller shaft is misaligned | Ensure that the spinner flask is well-centered on the magnetic plate and taped onto the plate in an optimal position prior to adding the alginate mixture. Test very high agitation rates and then decreased to the desired agitation rate prior to adding the alginate mixture. |
| Ensure that the impeller shaft is well-aligned and stable. For the Bellco spinner flasks, ensure that the impeller is firmly held in place by bolts on both sides of the spinner flask cap. Ensure that the impeller movement is limited to the radial direction (the impeller should not wobble on the shaft). | ||
| No beads obtained | The alginate was improperly emulsified | Ensure that alginate microdroplets are visible during the emulsification. If not, verify that the oil phase density and the vessel geometry23 are similar to those described in this protocol. A dense oil phase or vessel geometry that does not provide sufficient local energy dissipation may not allow proper alginate emulsification. |
| No beads obtained | The internal gelation did not occur | A common error is incomplete dissolution of the acetic acid in the oil phase. Ensure that the acid oil mixture is sufficiently vortexed and no acetic acid is observed at the bottom of the tube. |
If the impeller does not reach the oil, more oil can be added prior to adding the alginate. However, the amount of acetic acid should be adjusted according to the following equation: 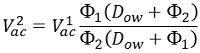 Where Where | ||
| No beads obtained, or beads are mechanically unstable | The alginate solution zero-shear viscosity is below 0.004 Pa·s or above 112 Pa·s | Due to the high batch-to-batch variability of alginate lots, it is recommended to measure the viscosity of different concentrations of alginate solutions. The combination of a high-viscosity batch with a low-viscosity batch may be required to reach a target viscosity. For the 5% LVM and MVG mixture used here, the zero-shear viscosity was 3.3 Pa·s. It should be noted that alginates with too short guluronic acid block lengths or short overall chain lengths may not allow gel formation. |
| Beads are too large or too small | The agitation rate during the emulsification is too low or too high for the alginate concentration or viscosity used | Increasing turbulence by increasing the agitation rate or adding baffles will decrease the bead size. The alginate concentration, the temperature, the vessel and impeller geometry will all impact the bead size obtained. |
| Many broken beads are observed | Harsh bead handling, damage during the internal gelation step or insufficient bead strength. | Mechanical damage to the beads can lead to bead rupture once internal gelation is initiated. Reduce the agitation rate immediately prior to the acidification step and ensure that the beads are handled gently by using 25 mL pipettes or 1000 µL pipettes with cut tips. If damaged beads are observed even with careful handling and reduced agitation during acidification, the bead strength may be inadequate. This could be due to insufficient guluronic acid content or incomplete gelling (e.g. insufficient acidification). |
| Low cell viability | Initial process pH too high or final pH too low | Mammalian cell process survival can be increased by limiting the pH drop and the acidification time23. However, these modifications may reduce the bead mechanical strength. Another potential issue may be the use of process solutions (e.g. alginate solution) with inadequately adjusted osmolalities. |
| Low cell viability in the smaller beads | Insufficient alginate solution buffering capacity | A greater pH drop is expected in the smaller beads than in the larger beads due to the greater surface/volume ratio of these beads. Since the acetic acid added to the emulsion is limited, higher acetic acid concentrations are predicted in smaller beads for the same CaCO3 concentration. Increasing the alginate solution buffering capacity (e.g. using 60 mM MOPS instead of 10 mM HEPES) can increase cell survival in the smaller beads32. Small beads with high cell losses could be selectively removed by filtration or sedimentation without incurring large volumetric losses. |
| Incomplete CaCO3 dissolution | Insufficient acidification or acidification time. Note that this issue may not be problematic if bead stability is sufficient. | Incomplete CaCO3 dissolution has been observed in the larger beads following this protocol32. The extent of CaCO3 dissolution can vary depending on the CaCO3 grain size, the pH drop during the process, the duration of the pH drop, as well as the alginate concentration used. This issue is not considered problematic as long as the bead mechanical stability is sufficient for the desired application. To increase bead cross-linking, ensure that the CaCO3 grain is sufficiently small (~2.5 µm). Sonicating the CaCO3 suspension can help disrupt aggregated grains26. At very low or high alginate concentrations, adjustments in the CaCO3 concentration may be required. Lastly, chelators or solutions with low levels of divalent ions will lead to the gradual loss of Ca2+ in the gel. If gradual loss of bead mechanical stability is observed, verify that the bead storage or culture buffer contains >2 mM Ca2+. |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.