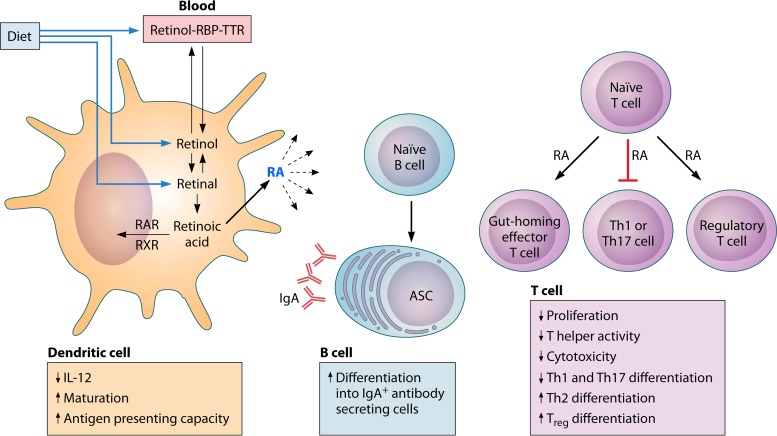
FIG 3.
Vitamin A metabolism and effect on immune cells in mucosa- and gut-associated lymphoid tissues. The fat-soluble vitamin A is acquired in the diet in the form of all-trans-retinol, retinyl esters, or β-carotene. These forms are solubilized in products of fat digestion and absorbed in micelles through the enterocyte membrane. Retinol circulates in the blood, complexed with retinol binding protein (RBP) and transthyretin (TTR). Retinol is oxidized to all-trans-retinal, which is then oxidized to all-trans-retinoic acid (RA) by retinal dehydrogenases, which are found in intestinal epithelial cells and gut-associated dendritic cells. Retinoic acid is exported from the cell and exerts autocrine and paracrine effects on immune cells by binding to nuclear receptors of the retinoic acid receptor (RAR) family, which heterodimerize with receptors of the retinoic X receptor (RXR) family. Together, these forms bind to retinoic acid response elements within promoters of retinoic acid response genes. In the presence of inflammatory stimuli, RA enhances dendritic cell maturation and antigen-presenting capacity. Dendritic cells also store and release RA to act on other immune cells. RA acts on naive T cells to upregulate the expression of gut-homing receptors. It reduces Th1 differentiation by blocking the expression of IL-12 by dendritic cells and T cell expression of the transcription factor Tbet and Th1 cytokines. It also blocks the induction of the transcription factor retinoic acid receptor-related orphan receptor γt (RORγt) and the differentiation of Th17 cells. In contrast, RA induces GATA3 and IL-4 expression, leading to enhanced Th2 differentiation, and promotes the differentiation of naive T cells to FoxP3+ regulatory T cells in intestinal tissue. B cells in mucosa- and gut-associated lymphoid tissues activated in the presence of RA differentiate into IgA+ antibody-secreting cells (ASC) (211).