Figure 1.
Engineering a Programmable dCas9-VP64-Based Signal Transduction Module
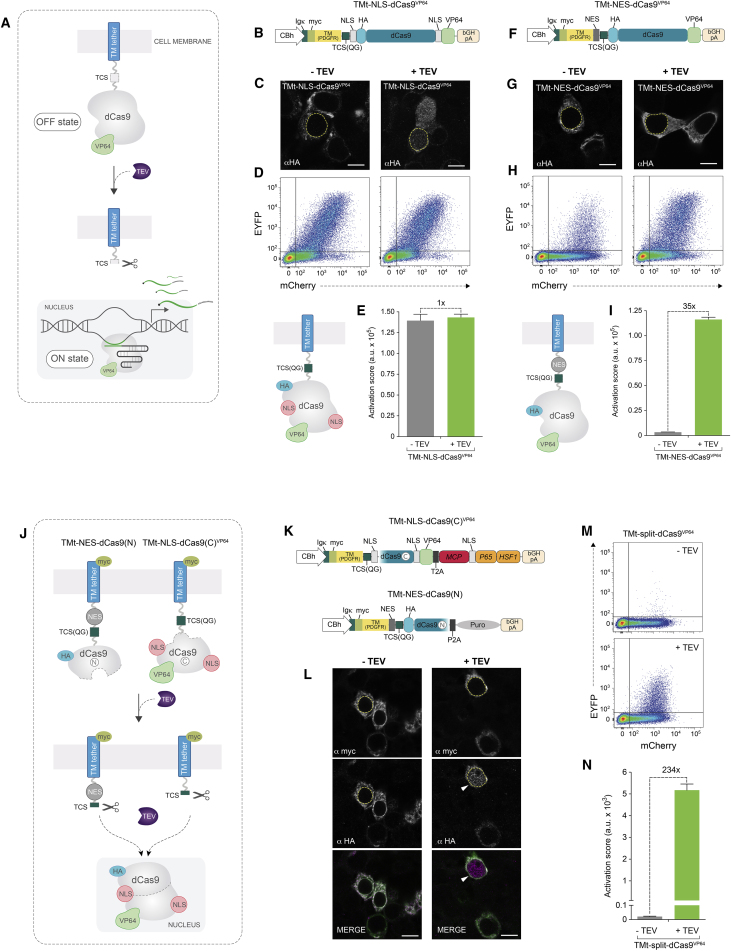
(A) Conceptual framework for the implementation of a basic CRISPR-TF membrane tethered module and TEV-based signal release mechanism.
(B) Molecular structure of the TMt-NLS-dCas9VP64 chimeric construct.
(C) Anti-HA confocal imaging of HEK293T cells transfected with TMt-NLS-dCas9VP64.
(D and E) TMt-NLS-dCas9VP64 system performance and OFF/ON state transition characteristics measured in the presence or absence of transgenic TEV protease. Representative flow cytometry scatterplots (D) and quantification of EYFP reporter activation score (E) 48 hr after co-transfection of plasmids encoding TMt-NLS-dCas9VP64, EYFP reporter, sgEYFP guide RNA, and TEV protease.
(F) Schematic representation of TMt-NES-dCas9VP64 variant.
(G) Immunofluorescence imaging of cells expressing TMt-NES-dCas9VP64 stained with anti-HA antibody.
(H and I) Representative flow cytometry scatterplots of reporter expression (EYFP channel) plotted against sgRNA transfection (mCherry channel) (H) and quantification of corresponding activation scores (see Experimental Procedures and Figure S1) (I). NES membrane tethered dCas9-VP64 variant baseline activation and fold induction following membrane release.
(J) Strategy for engineering a split dCas9-VP64 signal transduction module.
(K) Structure of split TMt-NES-dCas9(N) and TMt-NLS-dCas9(C)VP64 chimeric constructs. TMt-NLS-dCas9(C)VP64 plasmid contains the MCP-P65-HSF1 cassette to facilitate future implementation of endogenous gene expression programs.
(L) Confocal imaging of the constructs in (K) in the presence and absence of TEV protease, stained by anti-myc and anti-HA antibodies.
(M and N) Analysis of TMt-dCas9(N/C)VP64-induced reporter expression by flow cytometry (M) and quantification of corresponding EYFP activation score (N).
In all cases the EYFP activation score was calculated from three biological replicates (n = 3 from one experiment, mean ± SD; a.u., arbitrary units). For all confocal images, the dashed yellow line represents nucleus (based on DAPI staining). Scale bar, 10 μm. See also Figures S1 and S2.