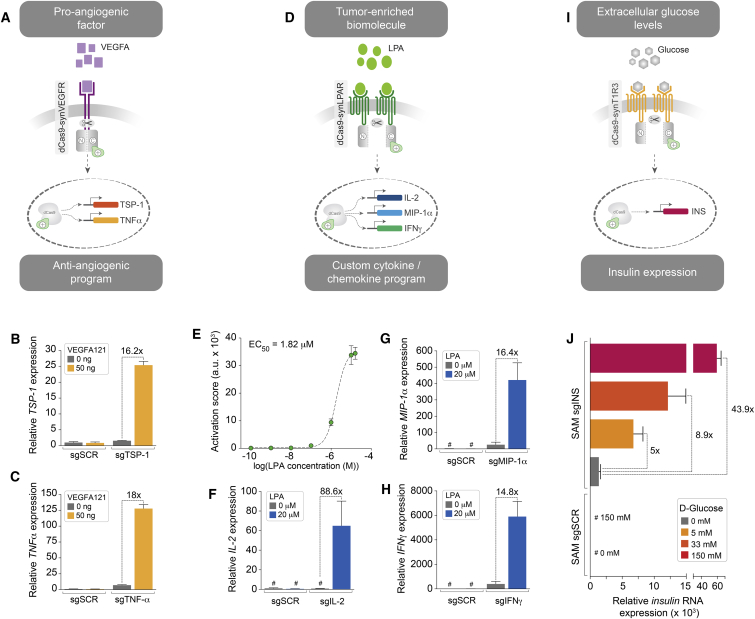
Figure 4.
Implementation of Prospective Therapeutic Programs with dCas9-synRs
(A) Conversion of a pro-angiogenic signal into a custom anti-angiogenic response by direct reprogramming of the optimized dCas9(N/C)-synVEGFR1/2 receptor with SAM sgRNAs for TSP-1 and TNF-α.
(B and C) Real-time qPCR analysis of TSP-1 (B) and TNF-α (C) in HEK293T cells expressing dCas9(N/C)-synVEGFR1/2 receptor and corresponding SAM sgRNAs, in the presence of VEGFA121 plasmid relative to no-agonist controls.
(D) LPA-mediated activation of a multifactorial cytokine/chemokine coordinated response in HTLA cells.
(E) Analysis of LPA dose-dependent induction of EYFP expression by dCas9(N/C)-synLPAR1 complemented with sgEYFP guide RNA (each data point represents EYFP activation score from 3 biological replicates, mean ± SD, a.u. arbitrary units; curve was fitted using a non-linear variable slope [four parameters] function in GraphPad Prism).
(F–H) Quantification of simultaneous dCas9(N/C)-synLPAR1-mediated activation of endogenous IL2 (F), MIP1α (G), and INFγ (H) genes in the presence of exogenously delivered LPA relative to no-agonist conditions.
(I) Coupling extracellular glucose levels with programmed insulin expression in HTLA cells.
(J) Quantification of insulin transcriptional activation by dCas9(N/C)-synT1R3 following delivery of increasing concentrations of glucose in HTLA cells. Real-time qPCR analysis shows dCas9(N/C)-synT1R3-mediated upregulation of insulin mRNA levels relative to OFF state (no agonist) at physiological glucose concentrations.
For all endogenous gene expression analyses n = 3 biological replicates (×3 technical replicates), mean ± SD; sgSCR, control SAM sgRNA; #, undetermined values for the gene of interest were set to a maximum Ct = 40 cycles.