Fig. 6.
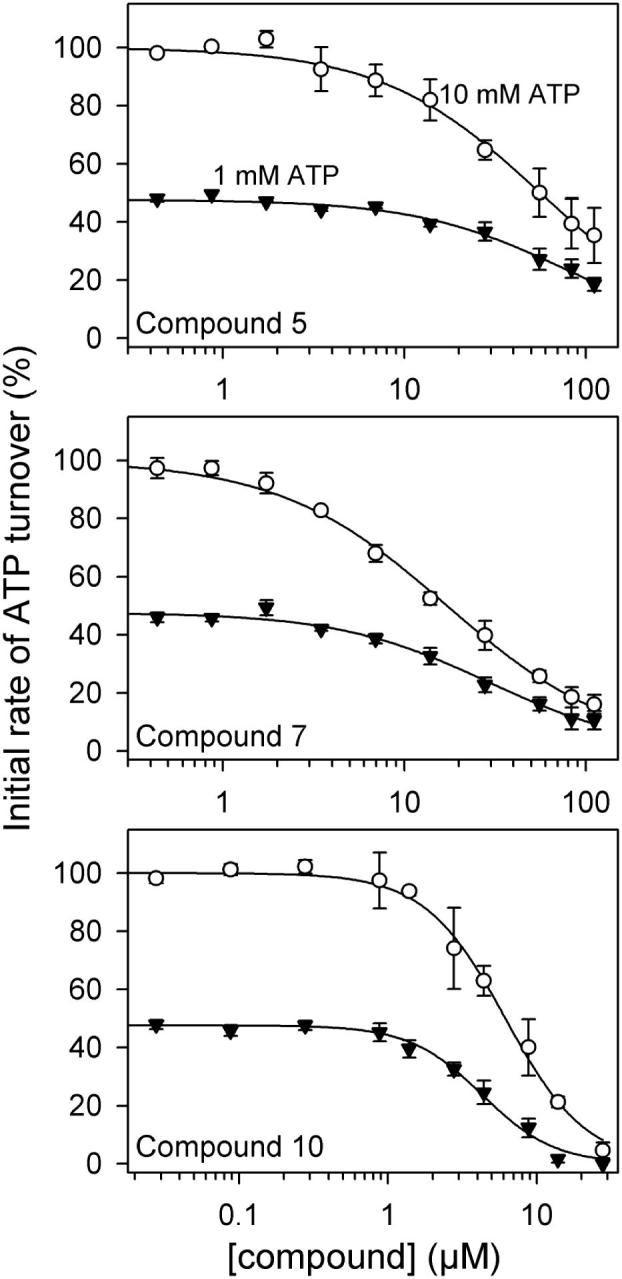
Compound dependence of the initial rate of H+-ATPase activity at 1 mM (triangles) or 10 mM (circles) ATP. The initial rates following compound addition deduced from experiments similar to those shown in Fig. 5 were normalized to the maximal rate obtained at 10 mM ATP in the absence of compound (presence of 1.1% DMSO) and plotted as a function of the compound concentration. The lines show the best fits of the equation V = Vmax · (1 − [cpd]h/(IC50h + [cpd]h)) to the data (n = 3), giving the following IC50 values: compound 5, 1 mM ATP, 78.0 ± 14.9 µM; compound 5, 10 mM ATP, 60.0 ± 14.2 µM; compound 7, 1 mM ATP, 29.7 ± 6.7 µM; compound 7, 10 mM ATP, 16.8 ± 1.7 µM; compound 10, 1 mM ATP, 4.5 ± 0.5 µM; compound 10, 10 mM ATP, 6.3 ± 1.2 µM.
