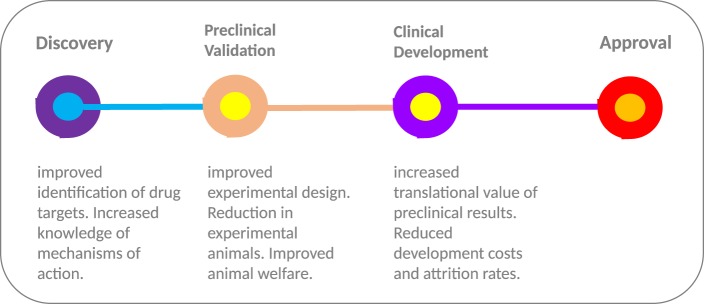
Figure 2.
From discovery to approval. The use of computational models throughout the drug development process provides the scope to improve experimental design and increase the translational value of early and preclinical stage results. The “models of models” approach provides a flexible test bed, enabling extended testing not feasible in animal models due to welfare and economic concerns. In combination with highly optimized computational models with more robust predictive capacity, the approach has the potential to increase the translational value of preclinical results and improve the high level of drug attrition rates, especially within the cancer arena.