Figure 1. Design of new ligands for Ni catalysis enables Suzuki coupling of benzylic acetals.
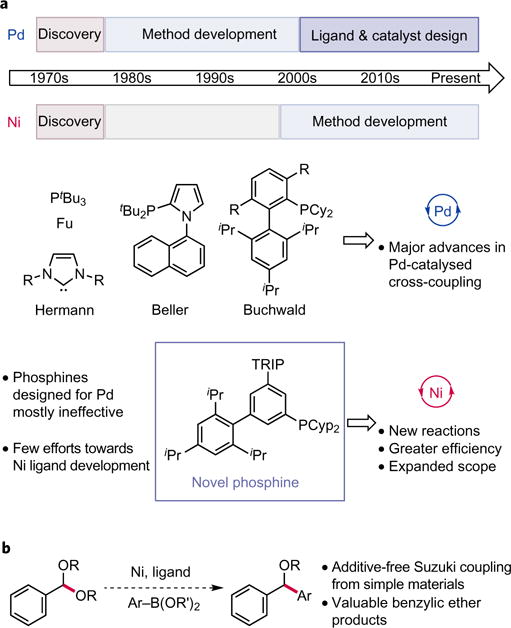
a, Ligand development in Pd versus Ni-catalysed cross-coupling. Ligands engineered for Pd catalysis have facilitated many advances in cross-coupling, including the discovery of altogether new transformations and the refinement of existing methods such that they can be used for large-scale synthesis. By contrast, minimal effort has been directed at ligand design for Ni-catalysed cross-coupling, despite the opportunity it presents for discovering novel reactions and for improving the efficiency of existing methods. b, Ligand design for Suzuki coupling of acetals. The development of a Ni-catalysed Suzuki coupling of acetals with readily available boronic acids would facilitate the preparation of valuable ether products by C–C bond formation. Application of known ligands for Ni and re-purposing ligands designed for Pd to this transformation were both unsuccessful in this regard, prompting the development and study of a new phosphine ligand class for Ni.
