Figure 2. Ligand evaluation and timepoint studies.
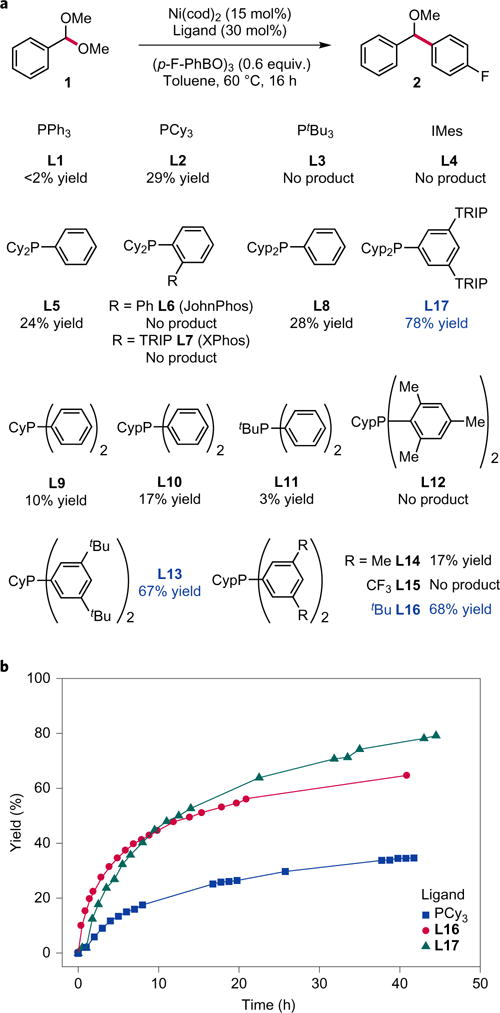
a, Screen of existing and novel phosphines for Ni. Ligand evaluation reveals that phosphines bearing tertiary alkyl groups or ortho-substituted aryl groups are completely ineffective. However, dialkylaryl [(alkyl)2PAr] and alkyldiaryl [(alkyl)PAr2] phosphines with secondary alkyl substituents and 3,5-substituted aryl groups are highly effective. IMes = 1,3-bis(2,4,6-trimethylphenyl)-imidazolium. Yields determined by 19F NMR with 2-fluorobiphenyl as a quantitative internal standard. b, Timepoint studies comparing the reaction profile with L16, L17 and PCy3 (L2). Ligands L16 and L17 afford highly active Ni catalysts in the Suzuki coupling; ligand L17 delivers the highest overall yields, despite a slower initial rate of reaction. Reactions were conducted at 60 °C in toluene (0.06 M), 15 mol% Ni(cod)2 and 30 mol% of ligand. cod = 1,5-cyclooctadiene.
