Table 2.
Scope investigation with more complex acetal substrates.
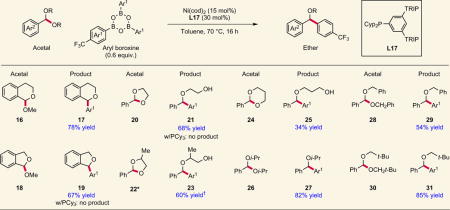
|
Several exocyclic and endocyclic acetals undergo C–C bond formation to afford valuable cyclic and acyclic ethers. Access to sterically hindered ethers is also possible using this method. In the cases examined, the new phosphine framework is exclusively effective. PCy3, a ligand that delivered low but measurable yield in the model reaction, is completely inactive, highlighting the impact of ligand development on reaction discovery. Yields shown are isolated yields. *1.3:1 mixture of diastereomers. †Run at 85 °C; isolated as a 2:1 mixture of regioisomers (major regioisomer shown).
