Abstract
Adolescence has been identified as a vulnerable developmental time period during which exposure to drugs can have long-lasting, detrimental effects. Although adolescent binge-like ethanol (EtOH) exposure leads to a significant reduction of forebrain cholinergic neurons, EtOH’s functional effect on acetylcholine (ACh) release during behavior has yet to be examined. Using an adolescent intermittent ethanol exposure model (AIE), rats were exposed to binge-like levels of EtOH from postnatal day (PD) 25–55. Three weeks following the final EtOH exposure, cholinergic functioning was assessed during a spontaneous alternation protocol. During maze testing, ACh levels increased in both the hippocampus and prefrontal cortex. However, selectively in the prefrontal cortex, AIE rats displayed reduced levels of behaviorally-relevant ACh efflux. We found no treatment differences in spatial exploration, spatial learning, spatial reversal, or novel object recognition. In contrast, AIE rats were impaired during the first attentional set shift on an operant set-shifting task, indicative of an EtOH-mediated deficit in cognitive flexibility. A unique pattern of cholinergic cell loss was observed in the basal forebrain following AIE: Within the medial septum/diagonal band there was a selective loss (30%) of choline acetyltransferase (ChAT) positive neurons that were nestin negative (ChAT+/ nestin−); whereas in the Nucleus basalis of Meynert (NbM) there was a selective reduction (50%) in ChAT+/ nestin+. These results indicate that early adolescent binge EtOH exposure leads to a long-lasting frontocortical functional cholinergic deficit, driven by a loss of ChAT+/ nestin+ neurons in the NbM, which was associated with impaired cognitive flexibility during adulthood.
Introduction
In 2015, approximately 5.1 million adolescents (12– 18 years old) reported binge alcohol use and 1.4 million adolescents reported heavy alcohol use within the past month (CBHSQ 2016). Alcohol exposure during adolescence has been tied to drug abuse vulnerability, as well as disruptions in cognitive functioning (DeWit et al., 2000; Jacobus and Tapert, 2013; Lisdahl et al., 2013). In rodents, exposure to ethanol (EtOH) during early adolescence has been linked to decreased behavioral inhibition and impairments in behavioral flexibility that are sustained into adulthood (Coleman et al., 2014; Gass et al., 2014; Doremus-Fitzwater and Spear, 2016). This distinctive behavioral phenotype results from sustained neural changes in critical brain regions such as the prefrontal cortex (Floresco et al., 2008; Fernandez et al., 2016; Vargas et al., 2014; Liu and Crews, 2015). Thus, early adolescent EtOH exposure produces an altered neural state that is associated with cognitive and memory impairments.
Adolescent intermittent ethanol (AIE) exposure models binge-like patterns of alcohol consumption (4 drinks for women/5 for men within 2 hours, resulting in blood alcohol concentrations above 0.08 g/dL) during adolescence (12– 18 years of age; (Spear and Swartzwelder, 2014). AIE has been associated with dysfunction in key learning and memory structures in adult rodents. Although AIE leads to decreased neurogenesis within the hippocampus (Geil et al., 2014; Vetreno and Crews, 2015; Sakharkar et al., 2016), AIE’s detrimental effects on spatial memory are inconclusive (Risher et al., 2013; Swartzwelder et al., 2015; Beaudet et al., 2016). Following AIE, there is also a significant loss in the number of cholinergic neurons within the medial septum/diagonal band (MS/DB), which projects to the hippocampus, and the Nucleus basalis of Meynert (NbM) that projects to the cortex (Ehlers et al., 2011; Vetreno et al., 2014; Boutros et al., 2015). These effects are age-specific; there is no difference in cholinergic populations following adult binge exposure models (Vetreno et al., 2014).
AIE also impacts the typical development of the prefrontal cortex (PFC), as there are persistent alterations in frontal cortical volume, myelination, neuroinflammation and, neurodegeneration (Crews and Nixon, 2009; Vetreno and Crews, 2012; Coleman et al., 2014; Vargas et al., 2014; Liu and Crews, 2015). These changes are proposed to contribute to deficits in behavioral flexibility, which result in an inability to readily adapt to changes in the environment (Dalley et al., 2004; Crews et al., 2016). Since adolescents typically demonstrate increased impulsivity and decreased behavioral inhibition compared to adults, AIE solidifies an adolescent phenotype into adulthood (Spear and Swartzwelder, 2014).
Although the neural correlates associated with AIE-mediated cognitive dysfunction have yet to be determined, the loss of forebrain acetylcholine (ACh) neurons is a key candidate. Within the rodent and human basal forebrain, there are two populations of cholinergic neurons (Hendrickson et al., 2011): All express choline acetyltransferase (ChAT), but about 35% of ChAT-expressing neurons also co-express nestin. Within the basal forebrain, nestin only co-localizes with ChAT, not with other neural populations (Hall & Savage, 2016; Hendrickson et al., 2011; Wang et al., 2006). As an intermediate filament protein, nestin is involved in the stabilization of the cell structure and remodeling (Michalczyk and Ziman, 2005; Gilyarov, 2008). The ChAT+/nestin+ neuronal subpopulation has unique electrophysiological properties: These neurons have higher excitability and receive stronger spontaneous excitatory inputs (Zhu et al., 2011). The role of nestin in cell structure, survival and plasticity has led to the hypothesis that the ChAT+/nestin+ phenotype may be neuroprotective for cholinergic neurons (Guo et al., 2010). ChAT+/nestin+ positive neurons also express nerve growth factor receptors at a high rate (95%; Guo et al., 2010), and typically have larger soma sizes within the MS/DB (Hendrickson et al., 2011). Indeed, the ChAT+/ nestin+ subpopulation within the MS/DB recovers following exercise-induced rescue from alcohol-related brain damage (Hall and Savage, 2016). Interestingly, the role of the ChAT+/ nestin+ subpopulation on AIE related dysfunction has not been explored.
Early adolescent exposure to high, binge-like levels of EtOH may cause cognitive impairment via alterations in cholinergic circuitry within the hippocampus and/or PFC. The current project examines AIE’s effects on spatial memory and cognitive flexibility, as well as its detrimental effects on cholinergic cell phenotypes and functionality. Peri-adolescent rats were exposed to binge-like levels of ethanol throughout the adolescent period and tested for behavioral deficits as adults. AIE selectively decreased PFC ACh levels during spontaneous alternation. Furthermore, AIE led to impairment in cognitive flexibility in an operant attentional set shifting task, as well as regional, unique cholinergic cell loss. ChAT+/ nestin+ cell estimates were decreased in the NbM, whereas ChAT+/ nestin− cell estimates were significantly decreased in the MS/DB. These results suggest the selective loss of the ChAT+/ nestin+ phenotype in the NbM contributes to deficits in PFC neurotransmitter release and associated cognitive deficit.
Experimental Procedures
Subjects
Young adolescent, male Sprague-Dawley rats were obtained from litters (postnatal day [PD] 10) shipped with dams (Envigo, Indianapolis, IN). Rats were randomly assigned into AIE (n=10) or control (n=13) groups. No more than one rat from each litter was randomly assigned within each treatment condition.
Rats were pair housed in a temperature (20°C) and humidity controlled colony under a 12-hour light/dark cycle (onset at 7:00 am). All experimental procedures were in compliance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the State University of New York at Binghamton.
Adolescent Intermittent Ethanol Exposure
Young adolescent rats (PD 25) were subject to 16 intragastric gavages of either 20% EtOH (v/v) or water, administered at a dose of 5 g/kg. The dosing schedule followed a 2-day on/off cycle, where rats were dosed once per day for 2 days, followed by a 2-day recovery period. This AIE schedule is a reproduction of previous protocols that have shown adolescent specific, EtOH mediated deficits on learning and memory (Liu and Crews, 2015; Vetreno et al., 2014; Vetreno and Crews, 2012, 2015) Blood samples were collected an hour following the first and eighth gavage and blood ethanol content (BEC) levels were determined using an AM1 Alcohol Analyzer (Analox Instruments). All animals gained weight during AIE treatment, and there were no significant differences in weight gain between treatment groups (F[1,21]=1.65, p>.20; data not shown).
After AIE, rats underwent cannula implantation surgery and recovery for a 3-week period. Figure 1 illustrates the AIE dosing and behavioral schedule. The sequence of behavioral testing was: Spontaneous alternation testing (with ACh microdialysis), novel object recognition, Barnes maze acquisition and reversal, and lastly, operant set shifting.
Fig. 1. Adolescent Intermittent Ethanol (AIE) Exposure protocol.
Schematic outlining the age range, AIE exposure protocol, and the timeline for cannula implantation surgery, behavioral testing and tissue collection (IHC: immunohistochemistry). Blood ethanol concentrations (BECs) following the first (1) and eighth (8) intraoral gastric gavage are illustrated in the chart insert. BECs did not significantly differ between the first and eighth gavage during AIE treatment.
Cannula Implantation Surgery
Hippocampal and PFC cannulations were performed on all rats 1.5 weeks following the cessation of AIE treatment. Prior to surgery, administration of a ketamine (10 mL)/xylazine (1.43 mL) mixture at a dosage of 50 mg/kg was administered (intraperitoneal) as anesthesia. Rodents were placed into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Guide cannula (HPC: 8 mm; PFC: 5 mm; Synaptech Technology Inc., Marquette, MI) were placed into the coordinates relative to Bregma: PFC: AP = + 2.7, ML = + 0.7, and DV = − 3.0 mm (Figure 3-A); HPC: AP = − 5.3 mm, ML = − 5.1 mm, and DV = − 4.2 mm (Figure 3-B). Dental acrylic cement with anchor bone screws secured guide cannula to the skull. Carprofen (5 g/kg; Zoetis, Kalamazoo, MI) was administered prior to surgery, as well as 24-hours and 48- hours post surgery as an analgesic. Rats recovered for 1.5 weeks before behavioral testing.
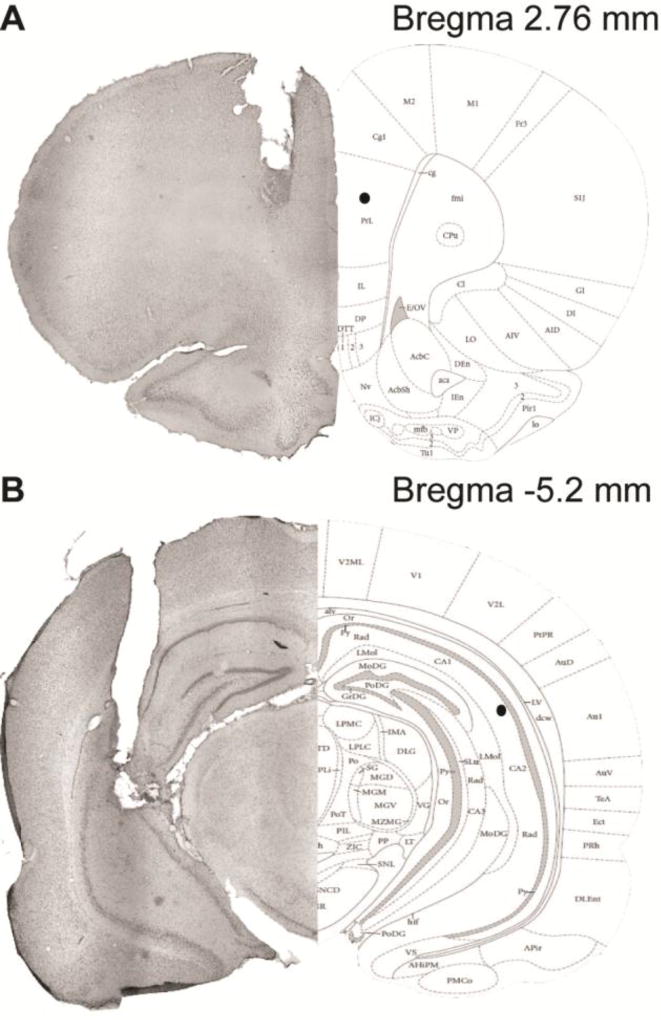
Fig. 3. Cannulae implant location.
Schematic demonstrating the typical location of cannula implantation (left panels: cresyl violet stain) for the prefrontal cortex (A) and hippocampus (B). The black dot on the right panels illustrate the target location for the end of the cannula (the probes extended 2 mm for the frontal cortex and 3 mm in the hippocampus). Right panel diagrams are taken from (Paxinos and Watson, 2013).
Spontaneous Alternation with in-vivo ACh microdialysis
Each rat was food restricted to 95% of his free feeding weight and was tested on a single spontaneous alternation session. Both ethanol and water pretreated animals were at similar weighs prior to food restriction, and all animals lost weight at similar rates. In-vivo microdialysis protocols were followed as previously described (Savage et al., 2003). On the day of testing, microdialysis probes (hippocampus: S-8020, 3 mm; PFC: S-5020, 2mm; Synaptech Technology Inc.) were inserted into the HPC and PFC guide cannula, respectively, and the rat was placed into an opaque habituation chamber to acclimate for a period of 60-min prior to maze testing. The probe was connected to a CMA microinfusion pump (CMA/400 pump) and an artificial cerebrospinal fluid solution (7.4 pH solution: 127.6 mM NaCl, 0.9 mM NaH2PO4, 2 mM Na2HPO4, 4 mM KCl, 1.3 mM CaCl2 dihydrate, 1.0 mM glucose, and 0.9 mM MgCl2) with 500 nM neostigmine hydrobromide (Sigma-Aldrich Corp.) was perfused continuously at a rate of 2.0 µL/min. Baseline dialysate collection began after the 60-minute acclimation period, and samples were collected for three 6-minute intervals.
Spontaneous alteration was conducted in a plus maze (105.5 cm × 14.4 cm × 15 cm). The rat was placed into the center of the apparatus, and allowed to explore the maze for 18-minutes of testing, during which arm entries (all four paws within an arm) were recorded. Dialysate was continuously collected during maze behavior. An alternation was defined as entry into four different arms in overlapping successive sequences of 4 arm entries (for example, in the successive arm entries of B, A, D, C, A, D, C, A, D, B, C, D, B, A; the first sequence of BADC was an alternation, but the next 4-arm sequence ADCA was not). The percent alternation score is equal to the ratio of actual alternations to possible alternations (total alternations/[trial number-3] × 100). In the example data set, the alternation score would be computed as follows: [4/(14-3)] × 100 = 36.4%. This criterion was adapted from previous experiments conducted in our lab (Fernandez et al., 2016; Hall and Savage, 2016). Following maze testing, the rat was returned to the opaque habituation chamber for an 18-minute collection of post maze dialysate.
High Performance Liquid Chromatography
Dialysate samples were assayed for ACh using high performance liquid chromatography (HPLC) with electrochemical detection (Eicom USA). ACh peaks were quantified by comparison to peak heights of standard solutions (100 nm, 20 nm, and 5 nm standards). Chromatographs obtained every 18 minute/sample were analyzed using the software program Envision (provided by Eicom, USA). Minimal detection was 5 femtomoles of ACh. Due to a blockage in one hippocampal probe, dialysate was collected from 9 EtOH pre- treated animals. The final n’s for collection in the PFC and hippocampus are noted on Figure 2-C and 2-D.
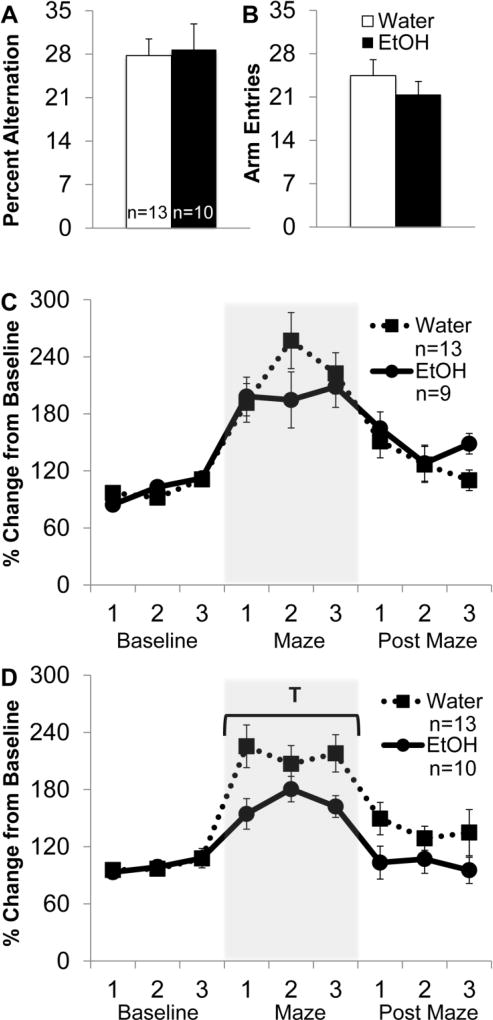
Fig. 2. AIE leads to blunted acetylcholine (ACh) efflux in the prefrontal cortex (PFC) during spontaneous alternation, but does not impair alternation behavior.
(A) Average percent alternation scores by AIE treatment. There was no effect of AIE on alternation behavior. (B) Arm entries during spontaneous alternation. There was no significant difference in activity between AIE and control groups. (C) Hippocampal ACh efflux before (baseline), during (maze) and after spontaneous alternation behavior (post maze). There was a main effect of phase, wherein ACh efflux significantly increased during maze behavior compared to baseline and post maze levels (p<0.05). AIE did not affect ACh efflux in the hippocampus. (D) Prefrontal cortical ACh efflux measured during baseline, maze behavior and post maze. There was a main effect of phase, with ACh levels increasing during maze behavior (p<0.05). There was also a significant interaction between AIE treatment and phase: AIE blunted the rise in ACh efflux during maze behavior in the PFC (T; p<0.05).
Novel Object Recognition
Testing parameters were adapted from Bevins and Besheer (2006). A black, apparatus, made in-house from high-density polyethylene, served as the arena (90 cm × 90 cm × 51 cm). The week following spontaneous alternation testing, rats were habituated to the arena for 10-minutes across a 3-day period. On the final day of habituation, a camera (Fujinon, YV5x2.7R4B-2) mounted to the ceiling recorded activity onto an automated tracking program system (Any-maze, Stoelting Company). Afterwards, during the familiarization phase, two identical objects (object A1 and A2) were placed into the left and right corners of a square testing apparatus equidistant from the walls and from each other. The rat was allowed to explore the objects for 5-minutes. The day following the familiarization phase (testing phase at 24-hours), one object used during familiarization was exchanged for a novel object the rat had not previously encountered (Object A1 and B). The rat was allowed to explore both objects for 5 minutes. We repeated the testing phase 7-days later with a different novel object (Object A2 and C). Object exploration was defined as having the rat’s head within 3 cm of the object. A discrimination index was computed for each rat and retention interval as follows: (seconds exploring the novel minus familiar object) /(seconds exploring the novel plus familiar object).
Barnes Maze
Testing parameters were adapted from (Vetreno and Crews, 2012). A black, circular table was made from high-density polyethylene (radius= 56 cm; 90 cm elevation). The table had 20 holes drilled alongside the circumference of the table at a 20° angle distance from each other (distance from border= 3 cm; radius= 5 cm). At any hole location, a rectangular goal box (10 cm × 37 cm × 14 cm) could be slid underneath as an escape hatch for the rat. Static noise from a radio filled the room, and floodlights illuminated the tabletop (lux>500). A camera (Fujinon, YV5x2.7R4B-2) mounted to the ceiling recorded activity onto an automated tracking program system (Any-maze, Stoelting Company, Wood Dale, IL), and the room was rich in extra-visual cues.
Testing consisted of three phases: training, reversal A and reversal B. Rats were placed inside an opaque box (25 cm × 34 cm × 12 cm) that was placed on a randomized quadrant of the maze. After 10 seconds, the rat was removed from the box and placed on the maze quadrant. During training, rats had 5-minutes to find the location of the goal box. If a rat failed to find the goal box, it was guided to the escape location by the experimenter. Latency to reach the goal box was recorded for each rat. The criterion for completion of the training phase was an average group latency of 30-seconds. After both treatment groups met criterion, the location of the goal box was switched 180° during reversal A. Each rat had up to 5-minutes to find the goal box, and training continued until criterion (group latency average <30-seconds) was met. After each rat met criterion, the goal box location was once again switched 180° for reversal B.
Operant Set Shifting
Testing parameters were adapted from (Brady and Floresco, 2015), with 11 operant chambers (30 cm × 33 cm × 23 cm; Med Associates Inc., St. Albans, VT) located inside sound-attenuating boxes (59 cm × 55 cm × 36 cm). Each box was outfitted with a fan in order to provide white noise. Each chamber had two retractable levers, one on each side of a magazine where a single food pellet (Rodent Purified Dustless Precision Pellet; Bio-Serve, Flemington, NJ) was delivered. There was a stimulus light positioned above each lever, and a house light was positioned in the top right corner of the wall opposite of the levers. Each chamber was interfaced to a computer program that integrated each testing session (MED-PC IV, Med Associates Inc.). Rats were food restricted to 85% of their free feeding body weight throughout the protocol. AIE and control rats did not have significant weight differences prior to food restriction, and all rats lost weight at similar rates (data not shown). The initial food restriction to 85% of free feeding weight occurred over 2 weeks.
The operant set shifting task consisted of five phases: a training phase during which each rat learned to lever press and its spatial (side) preference for a particular lever was determined; a response task during which the rat learned to lever press opposite of its side preference while a random light cue was to be ignored; a cue task (set shift 1) that required each rat to no longer use the spatial location (side preference) of the lever as the primary cue, but instead respond to the lever underneath the illuminated cue light. This was followed by a second response task (set shift 2) where the rat learned to ignore the cue light, and lever press opposite of its original side preference; and lastly a response reversal task, where the rat learned to respond to the opposite lever from the previous response task.
During the training phase, each rat was shaped to lever press on a fixed ratio 1 (FR1) schedule of reinforcement. At the beginning of the first training session, either the left or right lever was extended, with both the house and stimulus lights illuminated. Each lever press delivered a single food pellet. All rats underwent daily 30-minute training sessions until a stability criterion was met, defined as receiving at least 50 reinforced lever presses during a single session. Once stability was met, each rat was shaped on the opposite lever until stability criterion was met.
Retractable lever training followed shaping, where a single lever was presented per trial. The rat had to respond to the extended lever within 10-seconds of lever presentation, otherwise the lever retracted and an omission was counted. Presentation of the left versus right lever was pseudorandomized, with house and stimulus lights illuminated. The rat met stability criterion when it had less than 5 omissions per session over 2 consecutive sessions. The training phase ended with the determination of side bias. During side bias determination, each rat had to complete seven trials, and the lever that was responded to the most at the end of the session was assigned as the preferred lever/side preference.
Following side bias determination, response task (set acquisition) began over a series of sessions until the rat reached criterion, which consisted of responding to the rewarded lever 10 consecutive times. During the response task, the rat learned to respond to the lever opposite of its assigned side preference (spatial discrimination). The house light was illuminated during a trial, and a stimulus light (left or right, randomly determined to prevent association with a lever) was illuminated 3 seconds prior to lever presentation, which were presented for 10 seconds. Each rat had to perform a minimum of 30 trials, and there were a maximum of 200 trials per session. Each trial was presented every 20 seconds throughout the session.
After reaching criterion, a set shift was introduced (cue task) such that the rat had to learn to respond to the lever that is underneath the illuminated stimulus light. After the rat reached criterion on the cue task, a second set shift was introduced (response task), wherein the rat had to respond to the lever opposite its original side preference and no longer discriminate based on the stimulus light cue. After reaching criterion on the second set shift, a response task reversal was introduced. The rat had to switch its response to the lever opposite of what was previously rewarded (for example: left to right).
Trials required to reach criterion (10 consecutive rewarded lever presses), trial omissions, errors (pressing the non-rewarded lever) and total lever presses were analyzed for group differences.
Tissue Collection
A week following the final operant set shifting task, rats were euthanized (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI) and perfused (Masterflex Easy- Load Console Drive; 7518-00; Cole-Parmer Instrument Co.) with cold phosphate buffered saline and cold 4% paraformaldehyde (PFA; Electron Microscopy Services, Hatfield, PA), and post fixed for 24-hours in 4% PFA, followed by a 30% sucrose solution in 0.1 M PBS at 4°C until slicing. Brains were coronally sliced at 40 µm using a sliding microtome (Sm2000r Leica Biosystems, Wetzler, Germany). Tissue slices were maintained in a cryoprotectant solution (62.8 mg NaH2PO4, 2.18 g Na2HPO4, 160 mL dH2O, 120 mL ethylene glycol and 120 mL glycerol) at −20°C until immunohistological processing.
Brightfield ChAT/Nestin Double Labeling
Every 6th (MS/DB) or 7th (NbM) section per rat was processed for ChAT+/ nestin− and ChAT+/ nestin+ expression. On day 1, free-floating sections were washed using a standard 0.1 M Tris-buffered saline (TBS) solution (pH= 7.4). Next, sections were quenched using a 0.3% hydrogen peroxide solution for 30-minutes, followed by another TBS wash. Slices were then blocked in a solution consisting of 3% rabbit serum and 0.3% Triton X-100 in a 0.1 M TBS solution. Tissue was incubated overnight at 4 °C in ChAT primary antibody (AB144P; EMD Millipore; Billerica, MA; 1:200 dilution) made in blocking solution. On day 2, slices were washed in 0.1 M TBS and then incubated in a secondary antibody (biotinylated anti-goat IgG; BA-5000; Vector Laboratories; Burlingame, CA) with blocking solution. Tissue was washed in 0.1 M TBS and incubated in an avidin/biotin complex (VECTASTAIN Elite ABC HRP Kit, Vector Laboratories) for 1 hour in a solution of 0.1 M TBS and 0.3% Triton X-100. Tissue was then rinsed and developed using ImmPACT NovaRed Peroxidase solution (SK-4805; Vector Laboratories). Following the chromagen development, tissue was rinsed in 0.1 M TBS and blocked for 1-hour in a solution of 2.5% normal horse serum, 1% bovine serum albumin and 0.3% Triton X-100. Tissue was then incubated in the Nestin primary antibody (1:200 dilution; MAB353; EMD Millipore) in blocking solution overnight at 4 °C. On day 3, tissue was rinsed in 0.1 M TBS and incubated in a tagged secondary antibody solution (anti-mouse IgG polymer detection kit; MP-5402; Vector Laboratories) for 1-hour. Slices were washed in 0.1 M TBS and developed using Vector Blue Alkaline Phosphatase Substrate Kit (SK-5300; Vector Laboratories). After a final rinse in 0.1 M TBS, sections were mounted and cover slipped using VectaMount permanent mounting medium (H-5000; Vector Laboratories).
Cresyl Violet Staining
To determine the location of cannula implantation and probe insertion, PFC and HPC tissue was stained using a cresyl violet protocol (Paul et al., 2008). Briefly, sections were mounted onto slides and were rehydrated in a series of EtOH concentrations for 3 minutes (95%, 95%, 70%, 70%). Tissue was stained in cresyl violet (FD Cresyl Violet Solution; PS102-2; FD Neurotechnologies; Columbia, MD) for 5 minutes, rinsed in distilled water, differentiated in a series of EtOH concentrations for 2 minutes (70%, 95%, 95%), dehydrated in 100% EtOH for 2 minutes, and cleared in Citrisolv (04–355-121; Fisher Scientific) for 5 minutes. Slides were cover slipped using Permount mounting medium (SP15-500; Fisher Scientific).
Unbiased Stereological Counts of MS/DB and NbM Cells
The parameters for cell quantification were based on previously published studies (Hall and Savage, 2016; Roland and Savage, 2009; Savage et al., 2007). A Zeiss Microscope (Axioscope 2-Plus, Thornwood, NY) with an attached digital camera (DVC-1310; DVC Company, Austin, TX) containing a motorized stage in the x, y and z planes was used in conjunction with StereoInvestigator software (MicroBrightField Bioscience, Williston, VT) on a computer containing a Windows XP operating system. Using 5 × magnification, contours were drawn around the specific regions of interest (ROIs: MS, horizontal and vertical limbs of DB, NbM). Counting was performed using the optical fractionator function at 40 × magnification. Soma size was determined using the nucleator software function (rays=5). The section-sampling fraction was set at 1/6 for MS/DB sections and 1/7 for NbM sections. The counting frame was set at 50 µm × 50 µm, the grid size was 100 µm × 100 µm, the optical dissector height was 20 µm and the top guard zone was 2 µm. The section thickness was calculated and included to estimate the total number of neurons in the population (35 µm/ MS/DB section; 34 µm/ NbM section). The Gunderson–Jensen estimator of the error coefficient was lower than the recommended value of 0.10 (smoothness factor = 1.0; Gundersen and Jensen, 1987).
Within our ROIs, nestin is only expressed in ChAT+ neurons (Guo et al., 2010; Hendrickson et al., 2011), and the presence of nestin+/ ChAT- cells is undetectable with our current staining protocol. Therefore, immunopositive cells within the ROIs were coded as either ChAT+/ nestin− or ChAT+/ nestin+.
Experimental Design and Statistics
Analyses were performed in SPSS (IBM Corporation, Version 22). A one-way (Treatment: AIE vs. Control) analysis of variance (ANOVA) assessed spontaneous alternation and novel object recognition measures. Immunohistological data was analyzed using a one-way (Treatment: AIE vs. Control) ANOVA for both the total estimated cell population and cells per mm2 Both cell numbers and cell/mm2 were analyzed in order to verify that significant changes in cell populations were not due to changes in the forebrain nuclei size as a result of treatment. Repeated-measures ANOVAs, with Treatment as the between subjects factor, were used to analyze BEC (across time), Barnes Maze training and reversals, operant set shifting behavior (across tasks) and microdialysis output (phase × sample). Error bars on graphs, and variance reported with means, indicate the standard error of the mean.
Results
AIE rats reached binge-like levels of intoxication
BEC levels during AIE treatment remained stable across the first and eighth intraoral gastric EtOH gavage treatments (F [1,21]<1, p>0.10). Figure 1 illustrates the average AIE BEC values (≅160 mg/dL), which well exceeded binge EtOH benchmarks of 80 mg/dL (Spear, 2015).
AIE spared spatial memory assessed using a spontaneous alternation task
Analysis of percent alternation scores did not yield an effect of AIE (F[1,22]<1, p>0.10; Figure 2-A). Similarly, the number of arm entries during testing were not affected by prior AIE (F[1,22]<1, p>0.10; Figure 2-B).
AIE reduced ACh efflux in the prefrontal cortex, but not the hippocampus
Assessment of basal femtomole ACh levels in the hippocampus (AIE: x̅=36.6 ±4.7; Control: =48.6 ±6.2) and PFC (AIE: x̅=55.7 ±9.5; Control: =69.4 ±11.7) were not significantly different between AIE and control rats (F’s<1, p>0.20). Analysis of ACh efflux in the hippocampus indicated a main effect of phase (F[2,40]=48.4, p<0.001; Figure 2-C). Contrasts indicated that ACh efflux was significantly higher during spontaneous alternation testing in the hippocampus compared to baseline (pre-test) and post maze levels (F[1,21]=52.94, p<0.001). There was no interaction between AIE treatment and ACh efflux in the hippocampus (F[2,40]<2, p>0.10).
Analysis of ACh efflux in the PFC indicated a main effect of phase (F[2,42]=46.29, p<0.001; Figure 2-D). ACh efflux was significantly higher during spontaneous alternation behavior in the PFC compared to baseline and post maze levels (F[1,22]=73.35, p<0.001). There was also a significant treatment × phase interaction on PFC ACh efflux (F[2,42]=3.45, p<0.05). Due to the significant interaction, individual ANOVAs were run at each phase to determine the effect of AIE on behaviorally dependent ACh efflux. Results indicated that during spontaneous alternation maze behavior, AIE blunted ACh efflux compared to control levels (F[1,22]=5.2, p<0.05). There was also a trend for AIE rats to have blunted ACh levels post maze compared to control levels (F[1,22]=3.3, p=0.09).
Cannula Placement
Figure 3 illustrates the typical cannulation site for the PFC (Figure 3-A) and HPC (Figure 3-B). PFC cannulation sites targeted and hit the prelimbic cortex, which receives cholinergic afferents from the NbM; hippocampal cannulation sites targeted the CA1-CA3 regions, areas that receive cholinergic afferents from the MS/DB (Bloem et al., 2014; Teles-Grilo Ruivo and Mellor, 2013).
Cell Counting Regions of Interest
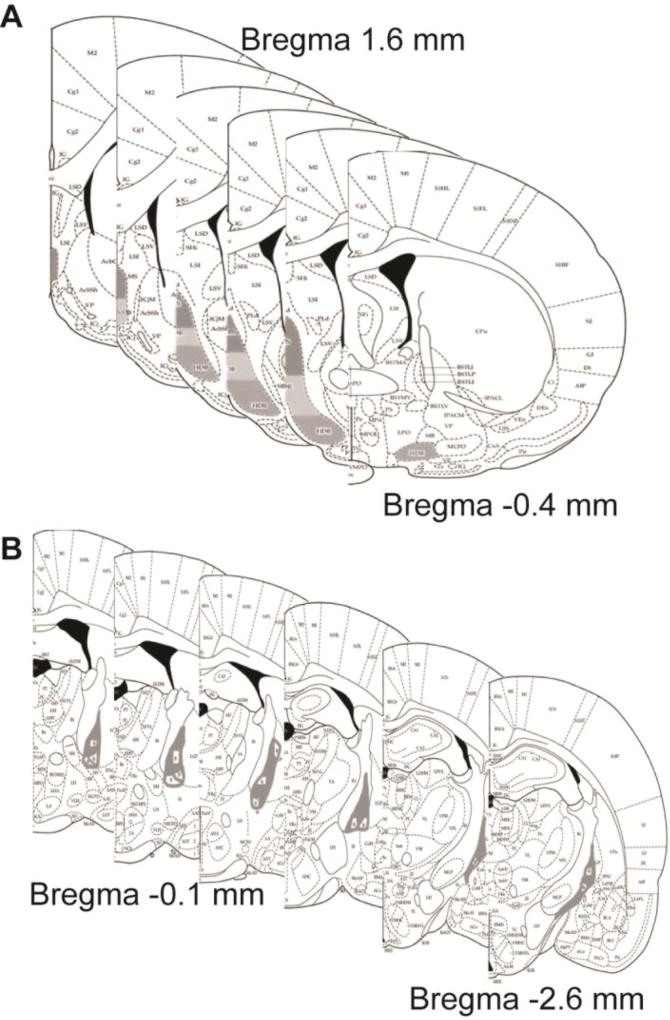
Figure 4 illustrates the areas included in both the MS/DB (Figure 4-A) and NbM cell counts (Figure 4-B). MS/DB contours included regions of the medial septum, as well as the vertical and horizontal limbs of the diagonal band, beginning at about 1.6 mm relative to Bregma and continuing through about −0.4 mm relative to Bregma, as outlined in Paxinos and Watson (2013). NbM contours began at about −0.96 mm relative to Bregma and continued through about −2.64 mm relative to Bregma, as outlined in Paxinos and Watson (2013). Stereological assessment required a semi-random start point for each region (West, 2013).
Fig. 4. Cell Counting Regions of Interest.
Schematic demonstrating representative cell counting contours for the medial septum/diagonal band (A), which began at 1.6 mm relative to Bregma and extended through −0.4 mm relative to Bregma. The light grey contours outline the medial septum, the dark grey outline denote the vertical limb of the diagonal band and the grey countours outline the horizontal limb of the diagonal band. Panel (B) illustrates the contours made for cell counts in the Nucleus basalis of Mynert, which extended from −0.96 mm to −2.64 mm relative to Bregna. All diagrams are taken from (Paxinos and Watson, 2013).
AIE spares recognition memory assessed using a Novel Object Recognition Task
After microdialysis, three control rats lost their head-gear and were removed from the study (final n’s are shown in Figure 5-A). Memory retention 24-hours and 7-days following the familiarization phase did not differ between AIE and control rats (F’s [1,18]<1, p>0.10; Figure 5-A). However, all rats explored the novel object at a rate greater than chance 24-hours after the familiarization phase (t(8)=2.56, p<0.05). This effect was not observed 7-days later. Additionally, there was no significant difference between treatment groups in the total time spent exploring objects during the 24-hour (AIE x̅=35.7 ±8.8 seconds, Control x̅=46.6 ± 7.3 seconds) and 7-day retention (AIE x̅= 29.9 seconds ± 6.5, Control x̅=38.4 ± 6.5 seconds) tests (F’s [1,19]<1, p>0.10).
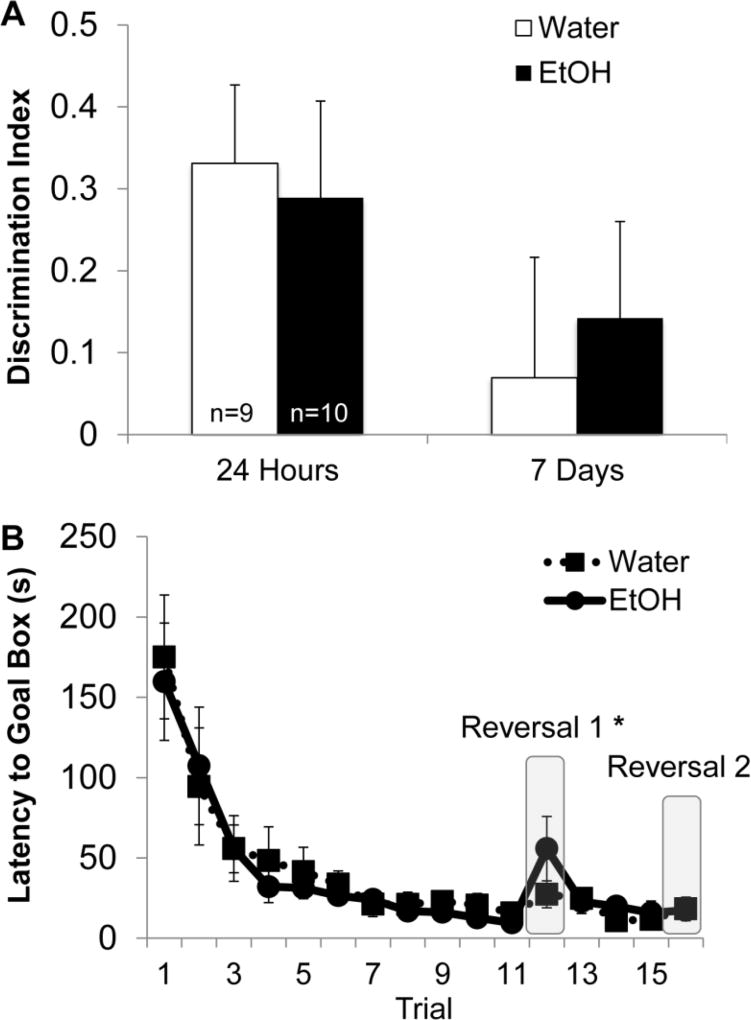
Fig. 5. AIE does not impair novel object recognition or Barnes maze behavior.
(A) Discrimination index (novel-familiar/novel+familiar) for the 24-hour and 7- day retention test. AIE did not significantly impair recognition memory at either delay. At 24-hours, both experimental groups spent more time exploring the novel object compared to the familiar object (*; p<0.05), an effect not seen at the 7-day retention test (with the discrimination index, a score of zero denotes no object preference). (B) Latency to reach the goal box during Barnes maze testing. Both experimental groups learned the spatial location of the goal box across 11 training trials. All rats took significantly longer to reach the new location of the goal box following the first reversal (*; p<0.05), an effect not seen following the second reversal. AIE did not significantly alter latency results during training or reversals.
AIE spared spatial and reversal memory assessed using a Barnes Maze
During training, both control and AIE rats learned the location of the goal box across 11 training trials (F[10,160]=18.17, p<0.001). However, AIE did not lead to significant differences in learning compared to control rats, (F[10,160]<1, p>0.10). After training, all rats took significantly longer to reach the goal box following reversal A (F[1,16]=10.4, p<0.01). AIE did not significantly affect the latency to reach the new location of the goal box following reversal A (F[1,16]<2, p>0.10). Additionally, there were no differences in the latency to reach the goal box from the final reversal A trial to reversal B (F[1,16]<1, p>0.10). AIE did not affect latency times during reversal B (F[1,16]<1, p>0.10). Figure 5-B shows the latency to reach the goal box across training and reversal trials.
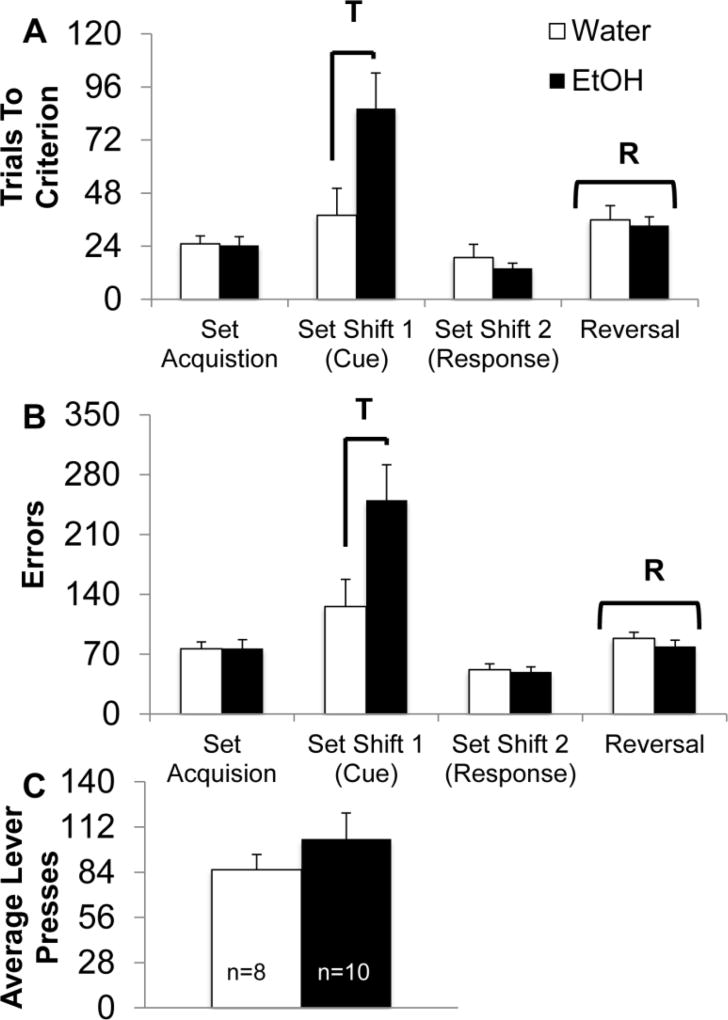
AIE increases the number of trials required to learn an attentional set shift
An additional control rat was removed from the study due to a loss of the microdialysis head-gear (final n’s are shown in Figure 6). Analysis of the trials required to reach criterion during operant set shifting indicated a main effect of set rule (F[3,48]=18.97, p<0.001; Figure 6-A) and an interaction between set rule and AIE treatment (F[3,48]=5.36, p<0.01). Follow-up analyses indicated that AIE rats took significantly longer to reach criterion on the first attentional set shift, compared to controls (F[1,17]=5.21, p<0.05; Figure 6-A). Additionally, all rats required more trials to reach criterion on the reversal task compared to the previous set task (F[1,16]=109.61, p<0.001), but there was no effect of AIE treatment on reversal learning (F[1,16]=1.2, p>0.10).
Fig. 6. AIE leads to an impairment in attentional set shifting.
(A) Average trials required to reach criterion (10 consecutive presses on the rewarded lever) during operant discriminations. AIE led to a significant increase in the number of trials required to reach criterion on the first set shift (response to cued discrimination; T; p<0.05). AIE did not affect rule learning, additional set shifts or a response reversal. All rats required significantly more trials to reach criterion after the response reversal (R; p<0.05). (B) Average errors (responding to the non- rewarded lever) committed during operant set shifting. AIE led to a significant increase in errors following the first set shift (response to cued discrimination; T; p<0.05). AIE did not affect rule learning, additional set shifts or a response reversal. All rats committed significantly more errors following the response reversal (R; p<0.05). (C) Average lever presses during operant set shifting. There were no significant group differences in lever pressing activity.
Analysis of the number of errors performed during operant set shifting indicated a main effect of set rule (F[3,48]=12.43, p<0.001; Figure 6-B) and an interaction between set rule and AIE treatment (F[3,48]=5.13, p<0.01). Follow-up analyses indicated that AIE rats made significantly more errors during the first attentional set shift, from response to cue discrimination, compared to controls (F[1,17]=5.2, p<0.05). We also found that regardless of treatment, all rats required more trials to reach criterion on the reversal compared to the previous set task (F[3,48]=12.43, p<0.001). We found no effect of AIE treatment on the average number of lever presses across set tasks (F[1,16]<3, p>0.10; Figure 6-C).
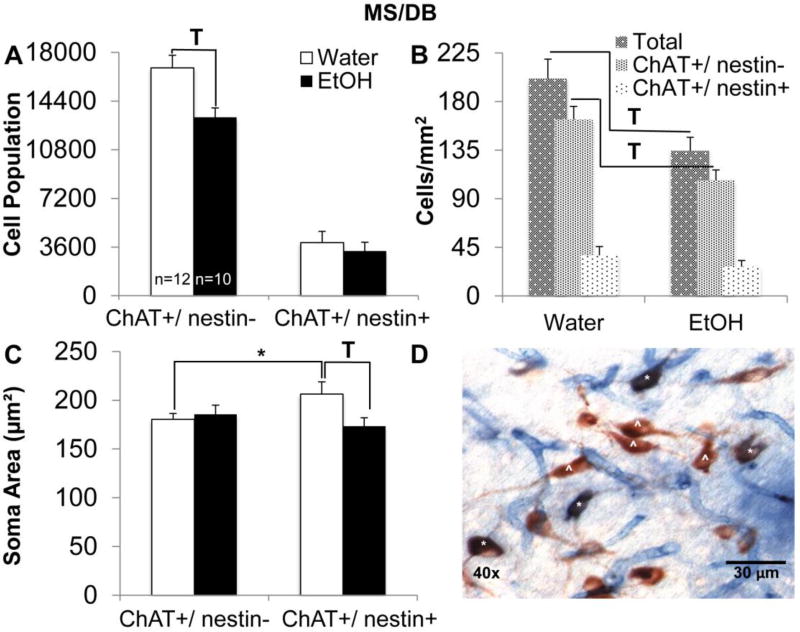
AIE reduced ChAT+/ nestin− cell populations in the MS/DB and ChAT+/ nestin+ cell populations in the NbM
AIE caused a significant decrease in the estimated ChAT+/ nestin− cell population within the MS/DB (F[1,21]=9.11, p<0.01; Figure 7-A). AIE did not affect the ChAT+/ nestin+ estimated cell population within the MS/DB (F<1, p>0.10). AIE also led to a significant reduction in the total number of cholinergic cells/mm2 of MS/DB tissue (F[1,21]=8.43, p<0.05; Figure 7-B), and a significant reduction in the number of ChAT+/nestin− cells per mm2 of MS/DB tissue (F[1,21]=12.76, p<0.05). There was no change in the number of ChAT+/ nestin+ cells per mm2 of MS/DB tissue as a result of AIE (F<2, p>0.10). However, AIE decreased soma area for ChAT+/ nestin+ cells within the MS/DB (F[1,21]=4.38, p<0.05; Figure 7-C), whereas no change in soma area was detected for ChAT+/Nestin− cells (F<1, p>0.10). When comparing baseline differences in soma area between the two cholinergic cell populations, control values for ChAT+/ nestin+ soma area were significantly larger than control values for ChAT+/nestin− soma area (t[11]= 2.34, p< 0.05).
Fig. 7. AIE leads to decreased choline acetyltransferase positive and nestin negative (ChAT+/ nestin−) cell populations and smaller ChAT+/ nestin+ soma areas in the medial septum/diagonal band (MS/DB).
(A) Estimated total number of ChAT+/ nestin− and ChAT+/ nestin+ cells in the MS/DB. AIE lead to a significant reduction in the estimated ChAT+/ nestin− cell population and did not affect the ChAT+/ nestin+ cell population in the MS/DB (T; p<0.05). (B) Number of cells (per mm2) in the MS/DB. AIE leads to a significant reduction in the total number of immunoreactive cells in the MS/DB (T; p<0.05), and a significant reduction in the number of ChAT+/ nestin− cells (T; p<0.05). AIE did not affect the number of ChAT+/ nestin+ cells. (C) Average soma area of ChAT+/ nestin− and ChAT+/ nestin+ cells in the MS/DB. AIE leads to a significant reduction in the soma area of ChAT+/ nestin+ cells in the MS/DB (T; p<0.05). ChAT+/ nestin+ soma areas were significantly larger compared to ChAT+/ nestin− soma areas in control rats (*; p<0.05). (D) Image depicting ChAT and nestin immunohistological stain at 40x magnification in the MS/DB. Representative ChAT+/ nestin− (^) cells are stained red and ChAT+/ nestin+ (*) cells are stained purple.
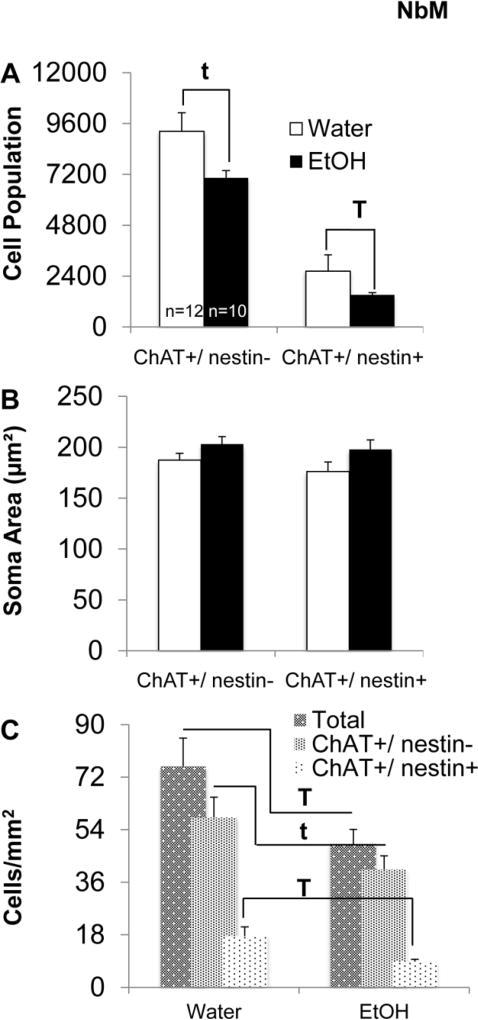
In the NbM, AIE caused a significant decrease in the estimated ChAT+/ nestin+ cell population (F[1,21]=8.21, p<0.01; Figure 8-A). There was a trend for an AIE mediated decrease in ChAT+/ nestin− cell population estimates (F[1,21]=3.4, p=0.08). AIE also led to a significant reduction in the total number of cholinergic cells/mm2 of NbM tissue (F[1,21]=5.27, p<0.05; Figure 8-B), and a significant reduction in the number of ChAT+/ nestin+ cells per mm2 of NbM tissue (F[1,21]=6.22, p<0.001). There was a trend in the reduction of ChAT+/ nestin− cells per mm2 of NbM tissue as a result of AIE treatment (F[1,21]=4.1, p=.06). Finally, in the NbM, there were no AIE-mediated differences in soma area for either ChAT+/ nestin− or ChAT+/ nestin+ cells (F’s<3, p>0.01; Figure 8-C).
Fig. 8. AIE leads to decreased ChAT+/ nestin+ cell populations in the Nucleus basalis of Meynert (NbM).
(A) Estimated total number of ChAT+/ nestin− and ChAT+/ nestin+ cells in the NbM. AIE significantly decreased the ChAT+/ nestin+ cell population in the NbM (T; p<0.05). There was a trend for AIE to decrease the estimated ChAT+/ nestin− cell population (t; p=0.08). (B) Number of cells (per mm2) in the NbM. AIE leads to a significant reduction in the total number of cholinergic cells in the NbM (T; p<0.05), and a significant reduction in the number of ChAT+/ nestin+ cells (T; p<0.05). There was a trend in the reduction of ChAT+/ nestin− cells per mm2 as a result of AIE (t; p=.06). (C) Average soma area of ChAT+/ nestin− and ChAT+/ nestin+ cells in the NbM. AIE did not +significantly affect soma area in either cell population.
Discussion
Our data revealed the novel finding that early adolescent binge-like EtOH exposure caused frontocortical cholinergic hypofunctionality as measured by ChAT+/ nestin+ cell loss in the NbM, blunted ACh efflux in the PFC. ACh efflux in the hippocampus during spontaneous alternation was not significantly altered by AIE, and this was paralleled by normal spatial learning and memory assessed by both spontaneous alternation and the Barnes maze. Furthermore, reversal learning in the Barnes maze and the operant set shifting task was not affected by AIE. Additionally, we did not find an effect of AIE on novel object recognition memory. However, AIE rats displayed a significant impairment in operant attentional set shifting. We also found an AIE mediated region-specific and phenotype- specific loss of forebrain cholinergic cell numbers. Specifically, AIE caused a reduction in ChAT+/nestin− cells within the MS/DB. However, in the NbM, there was a reduction in nestin positive, cholinergic cells (ChAT+/nestin+). We hypothesize this region-specific effect leads to frontal cortical dependent cognitive flexibility deficits that are dependent on cholinergic input from the NbM. These results provide more definitive evidence that cholinergic dysfunction within the frontal cortex is a critical mediator of alcohol-induced cognitive dysfunction that is associated with adolescent binge alcohol exposure.
There is converging evidence that spatial learning and memory are not severely affected by AIE. Similar to the findings presented here, we previously found that intermittent ethanol exposure during early and mid adolescence, as well as adulthood, did not affect spatial memory assessed by performance on a spontaneous alternation task (Fernandez et al., 2017). Additionally, AIE does not yield acquisition impairments on the Morris water maze, radial arm maze or the Barnes maze (Van Skike et al., 2012; Risher et al., 2013; Swartzwelder et al., 2014). In the current study, we also found spatial learning to be unaffected by AIE when assessed using a Barnes maze. These behavioral results are not in conflict with a loss of hippocampal neurogenesis, which occurs in AIE: When neurogenesis is ablated, acquisition progresses normally on the water maze and the radial arm maze, and novel object recognition is spared (Clelland et al., 2009; Jessberger et al., 2009). However, when task parameters are made difficult by closer spatial proximity, such that pattern separation is needed, loss of hippocampal neurogenesis does lead to behavioral impairment (Becker et al., 2017). Thus, intact neurogenesis is not required for all forms of hippocampal-dependent learning.
In contrast, it has been reported that reversal learning is negatively impacted by AIE, suggesting that behavioral inflexibility is a key behavioral deficit associated with alcohol-related brain damage. Vetreno and Crews (2012) found that AIE led to reversal impairments, as well as perseverative behavior, when the goal location was altered in the Barnes maze. The lack of reversal deficits, in both the Barnes maze and operant set shifting, following AIE in the current study could be a result of several factors. First, previous behavioral experience has been shown to affect later behavioral outcomes, suggesting that training can alter the neural systems supporting reversal learning (Rosenzweig and Bennett, 1996; Gould et al., 1999; Klintsova et al., 2002). Prior spatial testing, repeated reversal probes, and set shifting exposure in our current protocol may have served as the training necessary to circumvent orbitofrontal activation, thereby not yielding an AIE-induced reversal deficits. Although reversal learning is initially dependent on orbitofrontal activity (Bissonette et al., 2008; Ghods-Sharifi et al., 2008), performance on serial reversal tasks no longer requires intact orbitofrontal function (Boulougouris et al., 2007; Rich and Shapiro, 2007, 2009; Young and Shapiro, 2009).
It is important to note that our behavioral tasks were not counterbalanced. The sequence of behavioral testing was selected in order to accommodate cannula surgery and testing, as well as the timing required for operant set shifting training and food restriction. It is possible that the null effects in both the Barnes Maze task and novel object recognition task could be due to prior learning experience modulating later learning. Carryover effects are seen in both spatial memory tasks and recognition tasks (McIlwain et al., 2001; Cook et al., 2002; Cross et al., 2012; Hånell and Marklund, 2014; Paylor et al., 2006).
Second, strain differences have been reported on several measures related to EtOH dependency and toxicity (Priddy et al. 2017; Zahr et al., 2014), which may alter the behavioral response. Although AIE-dependent impairments in novel object recognition memory have been reported using a 24-hour intersession interval (Vetreno and Crews, 2015), these experiments were conducted in Wistar rats. Novel object recognition studies that employ Sprague Dawley rats either do not find age-specific deficits following binge EtOH exposure (Swartzwelder et al., 2012), or only do so after 24-hour interval between exposure and testing (Silvestre de Ferron et al., 2015). It is possible that the Sprague Dawley strain is not as sensitive to binge-level EtOH mediated recognition memory deficits, following drug-free recovery periods, compared to Wistar rats. Another issue could be the size of the testing arena and the spatial proximity between the objects, factors that influence whether the intact neurogenesis is critical for object recognition (Burke et al., 2010; Clelland et al., 2009; Antunes and Biala, 2012) . Interestingly, we did not see retention of recognition memory in either treatment condition after a 7-day intersession interval. It is possible that our parameters during the familiarization phase were not sufficient for long-term recognition memory induction in our rats (Vogel-Ciernia and Wood, 2014; Cohen and Stackman, 2015).
AIE did have a significant effect on performance during an operant attentional set shifting task, even after extended training. Attentional set shifting is an assessment of cognitive flexibility that requires the rat to alter the strategy or rule that governed a previous behavior across a task dimension, and inactivation or lesions to the PFC produce deficits in set switching (Birrell and Brown, 2000; Floresco et al., 2008; Rich and Shapiro, 2007, 2009). Our data complements previous research demonstrating that AIE leads to deficits in attentional set shifting (Gass et al., 2014). Both data sets indicate that AIE leads to deficits following a cognitive set shift from either visual to spatial cues (Gass et al., 2014) or from spatial to visual cues (current data). Previous research has indicated that a shift from spatial to visual task may be easier if a rat has no pre-exposure to the visual stimulus cue lights (Floresco et al., 2008). In our protocol, rats had pre-exposure to the stimulus cue lights during training, thereby circumventing the potential confound of novelty-induced approach (Brady and Floresco, 2015). Thus, the effect of AIE on cognitive set shifting is robust across cue shifts. Unlike reversal training, operant set shifting is less affected by prior reversal learning, suggesting that PFC and OFC are hierarchically organized to mediate different types of behavioral transitions (Young and Shapiro, 2009). In tandem with our data illustrating an AIE mediated blunting of prefrontal cholinergic responses during behavior, our results demonstrate that the early adolescent EtOH exposure specifically affects frontocortical functioning, while sparing hippocampal-dependent functioning.
The operant task used in the current experiment employed a rule change that required a shift in set from a location-based rule to a non-spatial rule in which the correct lever was indicated by a light cue. Our data complements previous research demonstrating that AIE leads to deficits in attentional set shifting (Gass et al., 2014). Interestingly, both data sets indicate that AIE leads to deficits following a cognitive shift from visual to spatial cues (Gass et al., 2014) and from spatial to visual cues (current data). Previous research has indicated that a shift from spatial to visual task may be easier if a rat has no pre-exposure to the visual stimulus cue lights (Floresco et al., 2008). In our protocol, rats had pre-exposure to the stimulus cue lights during training, thereby circumventing the potential confound of novelty-induced approach (Brady and Floresco, 2015). Additionally, our data suggest an AIE induced impairment in the first set shift, rather than a deficit in stimulus discrimination, because animals were not impaired on the second set shift, from cued discrimination to spatial. The animals in the current study demonstrated an ability to discriminate between the levers and cue signal, and EtOH- pretreated animals were only were impaired when they were required to learn a new rule.
A common finding of alcohol-related brain damage is a significant decrease (≈30%) in the cholinergic population within the forebrain nuclei (Savage et al., 2012; Vetreno et al., 2014, 2011; Swartzwelder et al., 2015). This reduction in basal forebrain cholinergic cell numbers is hypothesized to be a result of EtOH’s effects on neuroimmune activation that can lead to neural degeneration via oxidative stress or excitotoxicity (Crews et al., 2015). Interestingly, the decrease in cholinergic cell population within the MS/DB following AIE did not significantly alter ACh efflux within the hippocampus or impair spontaneous alternation and Barnes maze behaviors, which are ACh-dependent (Lalonde, 2002; Seeger et al., 2004; Anzalone et al., 2009). It is important to note that AIE only produces a decrease in the ChAT+/ nestin− population in the MS/DB. While our current data cannot indicate the mechanisms through which a decrease in MS/DB cholinergic cells did not lead to a decrease in functional hippocampal ACh release, there is the potential that the spared ChAT+/ nestin+ population within the MS/DB maintained ACh function within the hippocampus. ChAT+/ nestin+ cells, as mentioned previously, have higher spontaneous synaptic activity, which could potentially enhance cholinergic transmission (Zhu et al., 2011; Hall and Savage, 2016) and thus compensate for the loss of the ChAT+/ nestin− phenotype.
Interestingly, AIE did significantly decrease the average soma size of ChAT+/ nestin+ cells, but not ChAT+/ nestin− cells, within the MS/DB. Prenatal EtOH has been shown to negatively affect nestin and other proteins involved in neural structure (Taléns-Visconti et al., 2011; Veazey et al., 2013). These results indicate that early adolescent EtOH exposure may also affect the structure of this dynamic cholinergic cell population. In contrast, AIE’s effects on the ChAT+/ nestin+ phenotype in the NbM were more dramatic: Although alterations in soma size were spared, there was a dramatic reduction (50%) in the ChAT+/ nestin+ NbM cell population. Unlike the effects in the hippocampus, the AIE mediated ChAT+/ nestin+ cell population reduction in the NbM was concomitant with a decrease in frontocortical ACh efflux during maze testing. The hypofunctionality of ACh release in the PFC may be related to the deficits in cognitive set shifting seen in AIE treated rats, since cognitive flexibility is dependent on cholinergic functioning (Ragozzino, 2003; Logue and Gould, 2014; Prado et al., 2017).
However, the role of ACh in set-shifting is complex: Whereas NbM cholinergic lesions do not impair set shifting (McGaughy et al., 2008; Tait and Brown, 2008), modulation of nicotinic acetylcholine receptors enhances set shifting (Allison & Shoaib, 2013; Nikiforuk et al., 2015), and muscarinic receptor antagonism impairs set shifting (Soffie and Lamberty, 1987; Chen et al., 2004). Although the evidence from lesion studies suggest that ACh modulation of set-shifting behavior is extra-cortical (Allison and Shoaib, 2013), it likely that ACh interactions with multiple neurotransmitters across multiple cholinergic sites contribute to successful/unsuccessful cognitive flexibility (Prado et al., 2017). The AIE model does lead to loss of cholinergic neurons across the basal forebrain and brainstem projection systems, as well as a reduction of striatal cholinergic interneurons (Vetreno et al., 2014). The observed reduction of ACh efflux in the prefrontal cortex is likely one of several brain adaptions contributing to alcohol-induced cognitive impairment.
Previous studies have found that non- pharmacological treatments, such as exercise, can reverse both structural and functional deficits related to EtOH exposure (Thomas et al., 2008; Leasure and Nixon, 2010; Maynard and Leasure, 2013; Gallego et al., 2015). Exercise, via the upregulation of neurotrophins, could serve as a protective measure against EtOH’s effects on cognition by re-establishing normal cholinergic/nestin populations, well into adulthood (Gomez-Pinilla and Hillman, 2013; Vivar et al., 2013; Hall et al., 2014). Our laboratory has previously demonstrated that voluntary exercise specifically rescues cholinergic nestin subpopulation following alcohol related brain damage within the MS/DB (Hall and Savage, 2016). Although data is not currently available regarding the effects of exercise on NbM cholinergic/nestin, it is possible that this vulnerable nestin subpopulation would uniquely respond to exercise induced, neurotrophin mediated tropomyosin- related kinase receptor activation, which could result in normalized acetylcholine efflux within the frontal cortex (Hennigan et al., 2007; Lynch et al., 2013).
Our data demonstrate that high intoxication levels during early adolescence leads to a selective, compromised cholinergic phenotype within the NbM that is associated with a blunted functional release of ACh in the PFC that may contribute to cognitive flexibility deficits. Our AIE model was based on the characterization of adolescence that begins in the rat at PD 28 (Spear, 2000). Although other researchers have suggested that adolescence begins at a later time point, for example PD35 (Semple et al., 2013), our AIE model is consistent with previous research that has demonstrated adolescent specific deficits following EtOH exposure during PD28-PD55 (Ehlers et al., 2011; Broadwater and Spear, 2013; Vetreno et al., 2014; Vetreno and Crews, 2012). Additionally, our AIE model encompasses the peri-adolescent, early, mid and late adolescent periods that are in accordance with both Spear (2000) and Semple (2013). EtOH exposure throughout this early developmental time point, during which synaptic pruning, increasing myelination, and frontal cortical maturation occurs (Giedd et al., 1999; Tsujimoto, 2008; Tau and Peterson, 2010), has long lasting effects on the cholinergic system, as well as cognition.
Additionally, although our model did not employ an adult comparison group, previous studies have indicated the structural and functional deficits following intermittent binge EtOH exposure are an adolescent- specific phenomenon (Spear, 2011; Vetreno et al., 2014; Vetreno and Crews, 2015; Crews et al., 2016; Doremus-Fitzwater and Spear, 2016). Previous studies have found that regardless of potential age- related declines in cognition, binge EtOH exposure during adulthood does not typically lead to cognitive flexibility deficits (Coleman et al., 2014; Gass et al., 2014). Our findings demonstrate a mechanism by which the adolescent typical hypofunctionality of the PFC is maintained throughout development and into adulthood as a result of binge- like EtOH exposure (Spear and Swartzwelder, 2014).
Conclusion
Exposure to binge-like levels of EtOH that begins in peri-adolescence and extends through early adulthood leads to long-lasting frontocortical impairments. During spontaneous alternation behavior, PFC ACh levels were blunted following AIE. Our data suggest that this functional cholinergic deficit was driven by an AIE dependent decrease in NbM ChAT+/ nestin+ neuronal populations. Concomitant with hypofunctional cholinergic efflux in the PFC, we found cognitive flexibility deficits following AIE. Although we did not detect an effect of AIE on functional ACh efflux within the hippocampus, there was still an AIE dependent decrease in MS/DB ChAT+/nestin− cell numbers. This data suggest that the ChAT+/ nestin+ neuronal phenotype in the basal forebrain determines the functional cholinergic response to alcohol-related pathology.
Highlights.
Adolescent intermittent exposure leads to blunted acetylcholine release within the prefrontal cortex, but not hippocampus
Adolescent intermittent exposure leads to impaired attentional shifting, but spared spatial learning and memory
Adolescent intermittent exposure leads to a reduction of distinct in cholinergic populations within basal forebrain nuclei
Acknowledgments
This research was funded by a grant from the NIAAA to Lisa M Savage (RO1AA021775).
Abbreviation List
- ACh
Acetylcholine
- AIE
Adolescent intermittent ethanol
- ChAT
Choline acetyltransferase
- EtOH
Ethanol
- MS/DB
Medial septum/diagonal band
- NbM
Nucleus basalis of Meynert
- PFC
Prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison C, Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharm. 2013;64:314–320. doi: 10.1016/j.neuropharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone S, Roland J, Vogt B, Savage L. Acetylcholine efflux from retrosplenial areas and hippocampal sectors during maze exploration. Behav. Brain Res. 2009;201:272–278. doi: 10.1016/j.bbr.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet G, Valable S, Bourgine J, Lelong-Boulouard V, Lanfumey L, Freret T, Boulouard M, Paizanis E. Long-Lasting Effects of Chronic Intermittent Alcohol Exposure in Adolescent Mice on Object Recognition and Hippocampal Neuronal Activity. Alcohol. Clin. Exp. Res. 2016;40:2591–2603. doi: 10.1111/acer.13256. [DOI] [PubMed] [Google Scholar]
- Becker N, Kalpouzos G, Persson J, Laukka EJ, Brehmer Y. Differential Effects of Encoding Instructions on Brain Activity Patterns of Item and Associative Memory. J. Cogn. Neurosci. 2017;29:545–559. doi: 10.1162/jocn_a_01062. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory.”. Nat. Protoc. Lond. 2006;1:1306–11. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double Dissociation of the Effects of Medial and Orbital Prefrontal Cortical Lesions on Attentional and Affective Shifts in Mice. J. Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem B, Poorthuis RB, Mansvelder HD. Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front. Neural Circuits. 2014;8:17. doi: 10.3389/fncir.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav. Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int. J. Neuropsychopharmacol. 2015;18:2. doi: 10.1093/ijnp/pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Floresco SB. Operant procedures for assessing behavioral flexibility in rats. J. Vis. Exp. JoVE. 2015:e52387. doi: 10.3791/52387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav. Brain Res. 2013;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav. Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur. J. Neurosci. 2004;20:1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MN, Crounse M, Flaherty L. Anxiety in the elevated zero-maze is augmented in mice after repeated daily exposure. Behav. Genet. 2002;32:113–118. doi: 10.1023/a:1015249706579. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. Curr. Rev. 2015;37:331–351. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol. Rev. 2016;68:1074–1109. doi: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross L, Brown MW, Aggleton JP, Warburton EC. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn. Mem. 2012;20:41–50. doi: 10.1101/lm.028266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am. J. Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neurosci. Biobehav. Rev. 2016;70:121–134. doi: 10.1016/j.neubiorev.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, Stewart WN, Savage LM. Chronic Drinking During Adolescence Predisposes the Adult Rat for Continued Heavy Drinking: Neurotrophin and Behavioral Adaptation after Long-Term, Continuous Ethanol Exposure. PloS One. 2016;11:e0149987. doi: 10.1371/journal.pone.0149987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav. Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. Voluntary Exercise Decreases Ethanol Preference and Consumption in C57BL/6 Adolescent Mice: Sex Differences and Hippocampal BDNF Expression. Physiol. Behav. 2015;138:28–36. doi: 10.1016/j.physbeh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacol. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, Nixon K. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;54:103–113. doi: 10.1016/j.pnpbp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol. Learn. Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilyarov AV. Nestin in central nervous system cells. Neurosci. Behav. Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Hillman C. The Influence of Exercise on Cognitive Abilities. Compr. Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Guo K-H, Zhu J-H, Yao Z-B, Gu H-Y, Zou J-T, Li D-P. Chemical identification of nestin-immunoreactive neurons in the rat basal forebrain: a re-examination. Neurochem. Int. 2010;56:694–702. doi: 10.1016/j.neuint.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hall JM, Savage LM. Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior. Exp. Neurol. 2016;278:62–75. doi: 10.1016/j.expneurol.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Vetreno RP, Savage LM. Differential cortical neurotrophin and cytogenetic adaptation after voluntary exercise in normal and amnestic rats. Neuroscience. 2014;258:131–146. doi: 10.1016/j.neuroscience.2013.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hånell A, Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014;8:252. doi: 10.3389/fnbeh.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson ML, Rao AJ, Demerdash ONA, Kalil RE. Expression of Nestin by Neural Cells in the Adult Rat and Human Brain. PLoS ONE. 2011;6:e18535. doi: 10.1371/journal.pone.0018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem. Soc. Trans. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic Effects of Alcohol in Adolescence. Annu. Rev. Clin. Psychol. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Scamra C, Hoffman M, Napper RMA, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats:: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937:83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise Neuroprotection in a Rat Model of Binge Alcohol Consumption. Alcohol. Clin. Exp. Res. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to Delay? The Impacts of Adolescent Alcohol and Marijuana Use Onset on Cognition, Brain Structure, and Function. Front. Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Crews FT. Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience. 2015;293:92–108. doi: 10.1016/j.neuroscience.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Gould TJ. The Neural and Genetic Basis of Executive Function: Attention, Cognitive Flexibility, and Response Inhibition. Pharmacol. Biochem. Behav. 2014;123:45–54. doi: 10.1016/j.pbb.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a Novel Treatment for Drug Addiction: A Neurobiological and Stage-Dependent Hypothesis. Neurosci. Biobehav. Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ME, Leasure JL. Exercise Enhances Hippocampal Recovery following Binge Ethanol Exposure. PLoS ONE. 2013;8:e76644. doi: 10.1371/journal.pone.0076644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: Effects of training history. Physiol. Behav., Molecular Behavior Genetics of the Mouse. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol. Histopathol. 2005;20:665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Potasiewicz A, Popik P. Positive allosteric modulation of alpha 7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015;25:1300–1313. doi: 10.1016/j.euroneuro.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Paul CA, Beltz B, Berger-Sweeney J. The Nissl Stain: A Stain for Cell Bodies in Brain Sections. Cold Spring Harb. Protoc. pdb.prot4805. 2008 doi: 10.1101/pdb.prot4805. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Seventh Edition. 7. Academic Press; Amsterdam; Boston: 2013. [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol. Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Prado VF, Janickova H, Al-Onaizi MA, Prado MA. Cholinergic circuits in cognitive flexibility. Neuroscience. 2017;345:130–141. doi: 10.1016/j.neuroscience.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol. Learn. Mem. 2003;80:257–267. doi: 10.1016/s1074-7427(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J. Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher M-L, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, Markou A, Swartzwelder HS, Acheson SK. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PloS One. 2013;8:e62940. doi: 10.1371/journal.pone.0062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience. 2009;160:32–41. doi: 10.1016/j.neuroscience.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res., Synaptic Plasticity of the Cortex. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC. A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct. Funct. 2016;221:4691–4703. doi: 10.1007/s00429-016-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Chang Q, Gold PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn. Mem. 2003;10:242–246. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- Savage LM, Hall JM, Resende LS. Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychol. Rev. 2012;22:195–209. doi: 10.1007/s11065-012-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Roland J, Klintsova A. Selective septohippocampal - but not forebrain amygdalar - cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 Muscarinic Acetylcholine Receptor Knock-Out Mice Show Deficits in Behavioral Flexibility, Working Memory, and Hippocampal Plasticity. J. Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre de Ferron B, Bennouar K-E, Kervern M, Alaux-Cantin S, Robert A, Rabiant K, Antol J, Naassila M, Pierrefiche O. Two Binges of Ethanol a Day Keep the Memory Away in Adolescent Rats: Key Role for GLUN2B Subunit. Int. J. Neuropsychopharmacol. 2015;19 doi: 10.1093/ijnp/pyv087. pyv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffie M, Lamberty Y. Scopolamine disrupts visual reversal without affecting the first discrimination. Physiol. Behav. 1987;40:263–265. doi: 10.1016/0031-9384(87)90218-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Dev. Perspect. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci. Biobehav. Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Acheson SK, Miller KM, Sexton HG, Liu W, Crews FT, Risher M-L. Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers. PloS One. 2015;10:e0140042. doi: 10.1371/journal.pone.0140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher M-L, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48:353–360. doi: 10.1016/j.alcohol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder NA, Risher ML, Abdelwahab SH, D’Abo A, Rezvani AH, Levin ED, Wilson WA, Swartzwelder HS, Acheson SK. Effects of ethanol, Δ(9)-tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats. Neurosci. Lett. 2012;527:11–15. doi: 10.1016/j.neulet.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Taléns-Visconti R, Sanchez-Vera I, Kostic J, Perez-Arago MA, Erceg S, Stojkovic M, Guerri C. Neural differentiation from human embryonic stem cells as a tool to study early brain development and the neuroteratogenic effects of ethanol. Stem Cells Dev. 2011;20:327–339. doi: 10.1089/scd.2010.0037. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal Development of Brain Circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles-Grilo Ruivo LM, Mellor JR. Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 2013;5:2. doi: 10.3389/fnsyn.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav. Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Novier A, Diaz-Granados JL, Matthews DB. The effect of chronic intermittent ethanol exposure on spatial memory in adolescent rats: the dissociation of metabolic and cognitive tolerances. Brain Res. 2012;1453:34–39. doi: 10.1016/j.brainres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. J. Neurosci. 2014;34:14777–14782. doi: 10.1523/JNEUROSCI.3189-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey KJ, Carnahan MN, Muller D, Miranda RC, Golding MC. Alcohol-induced epigenetic alterations to developmentally crucial genes regulating neural stemness and differentiation. Alcohol. Clin. Exp. Res. 2013;37:1111–1122. doi: 10.1111/acer.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PloS One. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front. Neurosci. 2015;9:35. doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol. Learn. Mem. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. Curr. Top. Behav. Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Wood MA. Examining Object Location and Object Recognition Memory in Mice. Curr. Protoc. Neurosci. 2014;69:8.31.1–8.31.17. doi: 10.1002/0471142301.ns0831s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yao Z, Wang J, Ai Y, Li D, Zhang Y, Mao J, Gu H, Ruan Y, Mao J. Evidence for a distinct group of nestin-immunoreactive neurons within the basal forebrain of adult rats. Neuroscience. 2006;142:1209–1219. doi: 10.1016/j.neuroscience.2006.07.059. [DOI] [PubMed] [Google Scholar]
- West MJ. What to report: Information to be included in the publication of a stereological study. Cold Spring Harbor Protocols. 2013:815–819. doi: 10.1101/pdb.top071894. [DOI] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav. Neurosci. 2009;123:1028–1035. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hsu O, Vinco S, Orduna J, Luong R, Bell RL, Sullivan EV, Pfefferbaum A. Rat strain differences in brain structure and neurochemistry in response to binge alcohol. Psychopharmacology (Berl.) 2014;231:429–445. doi: 10.1007/s00213-013-3253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Gu H, Yao Z, Zou J, Guo K, Li D, Gao T. The nestin-expressing and non-expressing neurons in rat basal forebrain display different electrophysiological properties and project to hippocampus. BMC Neurosci. 2011;12:129. doi: 10.1186/1471-2202-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]