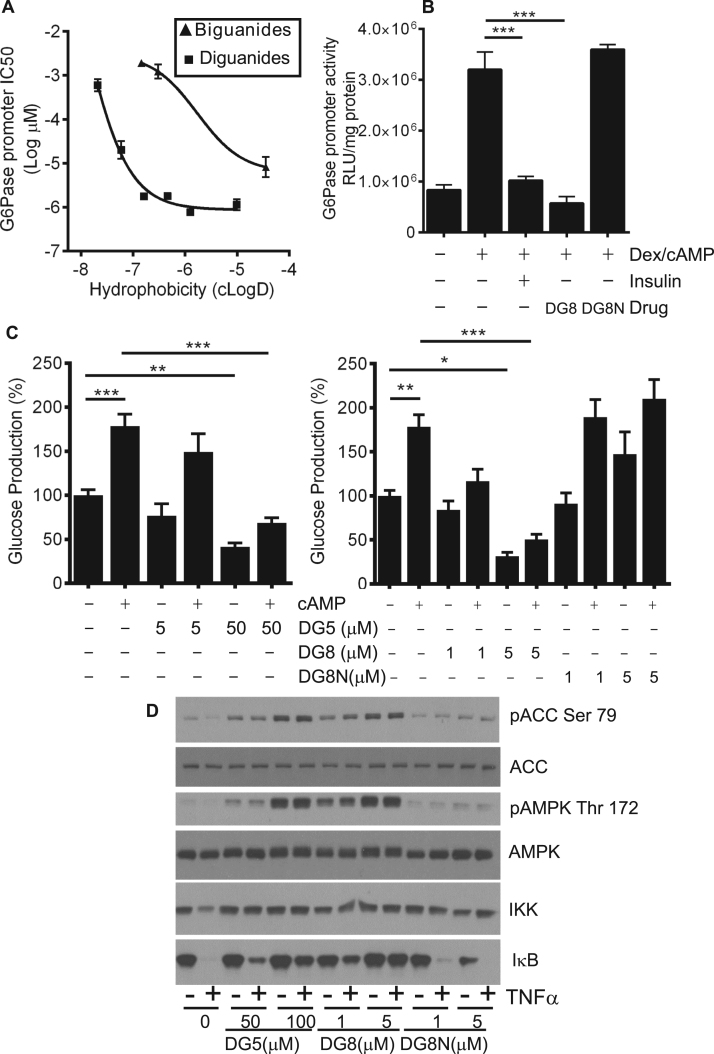
Fig. 3.
Relationship between EC50 on G6Pase promoter activity, hepatocyte glucose production and hydrophobicity of the drugs.(a) IC50s were determined for each drug on G6Pase promoter activity using LLHG cells. Calculated hydrophobicity for each compound was also determined. The relationship between log IC50 and clogD is presented. Plot of (left to right) biguanide, metformin and phenformin (triangles) and (left to right) DG4, DG5, DG6, DG7, DG8 and DG10 (squares). A lower log IC50 indicates a more potent compound, these values are tabulated in Table 2. Hydrophobicity plots of selected structures are provided in supplementary Figs. 3 and 4. (b) Comparison of 1 μM DG8 and 1 mM DG8N on G6Pase promoter activity. Dex/cAMP was used to stimulate the promoter. Ins denotes insulin. In (a) and (b) effects of each dose were measured at least six times, each from a separate well of cells. (c) Primary hepatocytes were treated in the presence or absence of DG5, DG8 and DG8N with and without 100 µM cAMP and glucose production measured as described in the Methods. A one-way ANOVA was carried out. (d) Primary hepatocytes were serum starved overnight and then were treated with the diguanides shown for 3 h, prior to 15 min treatment with TNFα before lysis and SDS-PAGE as detailed in the Methods. Immunoblotting was performed using antibodies described in Fig. 1. In addition, data is shown from an antibody for IKKα and one for IκB. The phosphorylation and protein degradation responses for IκB are representative of observations from at least three similar experiments. Quantitation of these replicates by densitometry appears in Supplementary Fig. 5.