Figure 7. ER stress responses is involved in apoptosis of Mtb infected macrophages from Sp110 transgenic cattle.
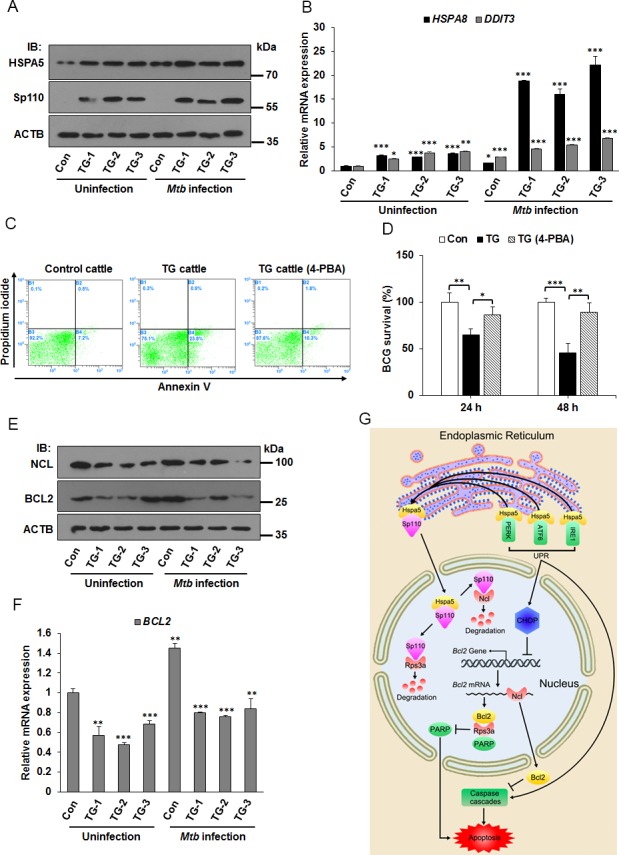
(A) Sp110 induces ER stress marker Hspa5. Macrophages isolated from control cattle and Sp110 transgenic cattle were infected with BCG (MOI 5:1) for 24 h. The expression of Sp110 and Hspa5 was determined by immunoblots. Con, macrophages from control cattle. TG, macrophages from Sp110 transgenic cattle. (B) Total RNA was extracted from macrophages of (A). The expression of HSPA8 and DDIT3 was determined by qPCR. (C) Macrophages were isolated from control cattle and Sp110 transgenic cattle, and then challenged with BCG (MOI 5:1) for 24 h. The TG cattle (4-PBA) sample was pretreated with 4-PBA for 1 h before Mtb infection. 4-PBA remained for the rest of the infection. Cell apoptosis was quantified by Annexin-V staining followed by flow cytometric analysis. (D) Macrophages isolated from Sp110 transgenic cattle were pretreated with 4-PBA for 1 h, and then infected with BCG (MOI 5:1) for 24 h and 48 h. 4-PBA remained for the rest of the infection. Intracellular mycobacterial number was determined by CFU counting at the indicated timepoints. (E) and (F) Sp110 inhibits BCL2 expression in bovine macrophages. Samples were prepared as (A), the protein levels of NCL and BCL2 were detected by immunoblots (E) and the mRNA of BCL2 was determined by qPCR (F). (G) Model of Sp110-mediated macrophage apoptosis. Sp110 competitively binds to Hspa5, which translocates Hspa5 into the nucleus to activate ER stress and initiate the apoptotic pathway. Moreover, Sp110 promotes protein degradation of Ncl and Rps3a, downregulating the anti-apoptotic Bcl2 expression and upregulating PARP activity, thus promoting apoptosis. Data are present as means ±SD of three independent experiments,* p< 0.05, **p< 0.01, and *** p< 0.001.
