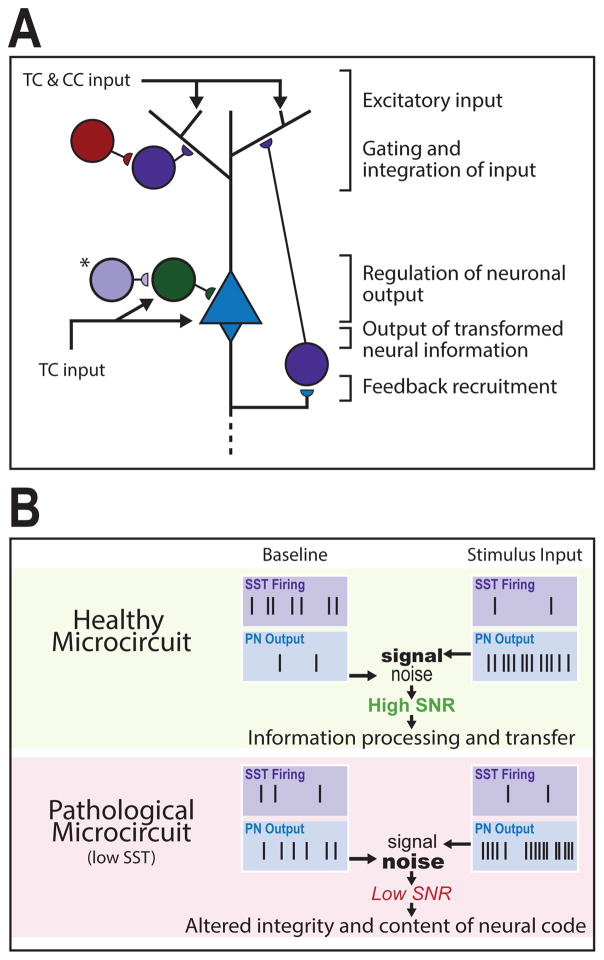
Figure 1. Cortical microcircuit roles and pathological consequences of low SST cell function.
(A) Excitatory signals originate through thalamocortical (TC) and cortico-cortical (CC) projections that converge upon pyramidal neurons (PNs). Somatostatin-expressing interneurons (SST interneurons) primarily regulate gating and integration of dendritic input through PN feedback recruitment. Vasoactive intestinal peptide-expressing (VIP) interneurons inhibit supragranular SST interneurons to facilitate excitatory input. TC input targets the PN soma and parvalbumin-expressing (PV) interneurons simultaneously, generating low-frequency PN output that is regulated by PV feed-forward inhibition. (*), In layer 4 a distinct SST interneuron population targets PV interneurons for somatic disinhibition of PN output. Dendrite- and soma-level activity combines to drive PN output, which is propagated across cortical layers and brain regions. (B) In a healthy microcircuit (top) at baseline, SST interneurons exhibit high spontaneous activity that maintains sparse PN activation. Upon stimulus input, SST interneurons reduce firing (e.g. through VIP-SST inhibition) resulting in sustained and ordered bursts of PN output. The difference between activated and baseline PN output patterns represents a signal-to-noise ratio (SNR) that facilitates coherent information processing and transfer. In a pathological microcircuit (bottom), low SST function is predicted to result in increased PN baseline activity (i.e., increased noise) and decreased regulation of stimulus-induced PN firing (i.e., altered signal) through altered input gating, disynaptic inhibition, and other disinhibitory roles, hence leading to low SNR and translating into altered integrity and content of neural code.