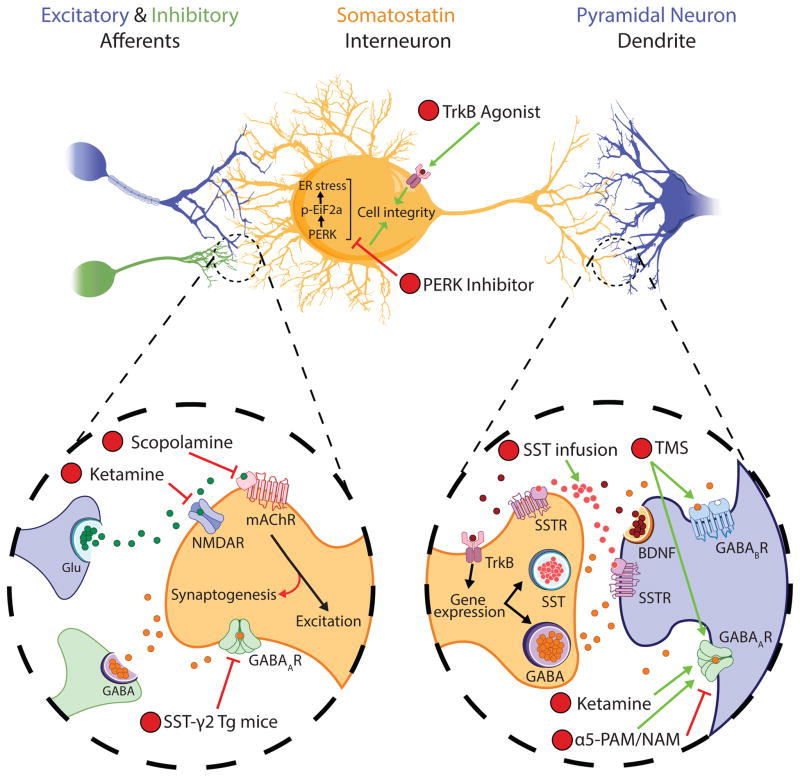
Figure 2. Targeting SST-expressing GABA interneuron signaling as an antidepressant strategy.
(1) Preclinical studies demonstrate that antidepressant-like effects of the rapid-acting antidepressant scopolamine depends upon expression of mAChRs specifically in mPFC SST interneurons (134). (2) Further evidence suggests that the antidepressant-like effects of the NMDAR antagonist, ketamine (2), may similarly occur through potentiation of GABA interneuron function. These drugs may converge on antagonism of receptors that drive excitatory activity of SST interneurons, resulting in a rapid cortical glutamatergic surge (through acute inhibition of SST interneurons) that feeds-back on a longer time-scale to promote SST interneuron function (e.g. through synaptogenesis or potentiation of α5-GABAA receptor (α5-GABAAR) function (135, 136)). (3) Similarly, transgenic (Tg) mice heterozygous for SST interneuron γ2-GABAAR-subunit knockout demonstrate an antidepressant- and anxiolytic-like behavioral profile, putatively resulting from disinhibition of SST interneuron function (112). Experimental compounds that promote SST cell integrity, e.g., by increasing brain-derived neurotrophic factor (BDNF)-TrKB signaling (4) or inhibiting protein kinase RNA-like endoplasmic reticulum (PERK) and therefore endoplasmic reticulum (ER) stress (5), have shown antidepressant-like action in rodent stress models (85, 149). (6) Interventions that potentiate the post-synaptic modulators of SST interneuron inhibition also show potential for monitoring and remediating SST interneuron deficits. Notably, pharmacologic α5-GABAAR positive and negative allosteric modulation (α5-PAM/NAM) show antidepressant-like activity in mice (139, 146). (7) In human, transcranial magnetic stimulation (TMS) has efficacy in patients with treatment-resistant depression (45), and was recently demonstrated in rodent to induce cortical inhibition through recruitment of dendrite-targeting supragranular interneurons acting through GABAAR- and GABABR-mediated neurotransmission, putatively implicating SST interneurons in the antidepressant effects of TMS (125). (8) Finally, direct infusion of SST or SST agonists into corticolimbic brain regions produces antidepressant-like effects in rodents (87–90). Glu, glutamate; GABA, γ-aminobutyric acid; NMDAR, N-methyl-D-aspartate receptor (NR2B subunit-containing); mAChR, muscarinic acetylcholine receptor (m1 subtype); p-Eif2a, phosphorylated eukaryotic initiation factor 2α; TrKB, tropomyosin receptor kinase B; SSTR, somatostatin receptor (pre- and post-synaptic).