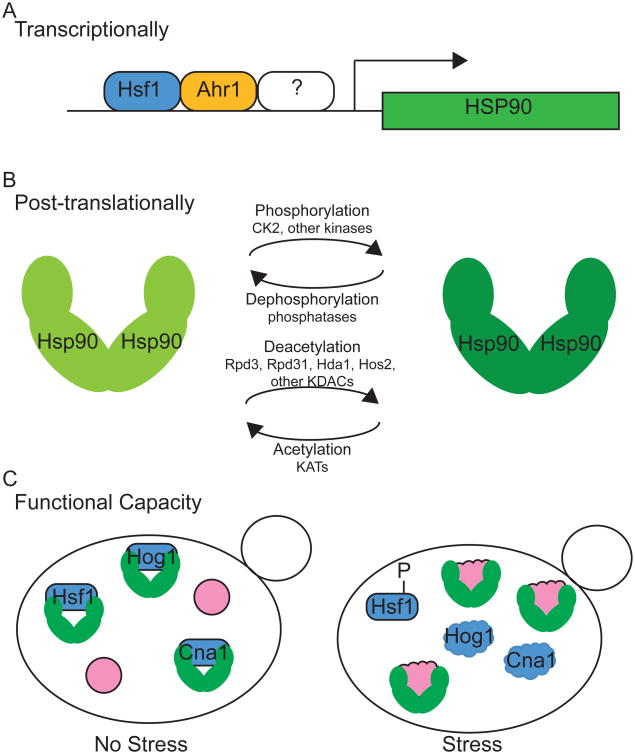
Figure 2. Models of Hsp90 regulation in C. albicans.
Hsp90 has been found to be regulated at the transcriptional and post-translational levels in response to environmental signals, with Hsp90 functional capacity modulated by cellular stress. A. HSP90 transcription can be regulated by Hsf1 and Ahr1, in addition to other factors. B. Hsp90 can be post-translationally modified by phosphorylation and acetylation. The phosphorylated and deacetylated form is required for functions in client stabilization and drug resistance. The phosphatase and lysine acetyltransferases (KATs) involved have not been elucidated. C. Increased cellular stress can overwhelm the functional capacity of Hsp90, impairing its ability to chaperone specific client proteins. Unfolded proteins are represented by ‘cloud’ shapes.