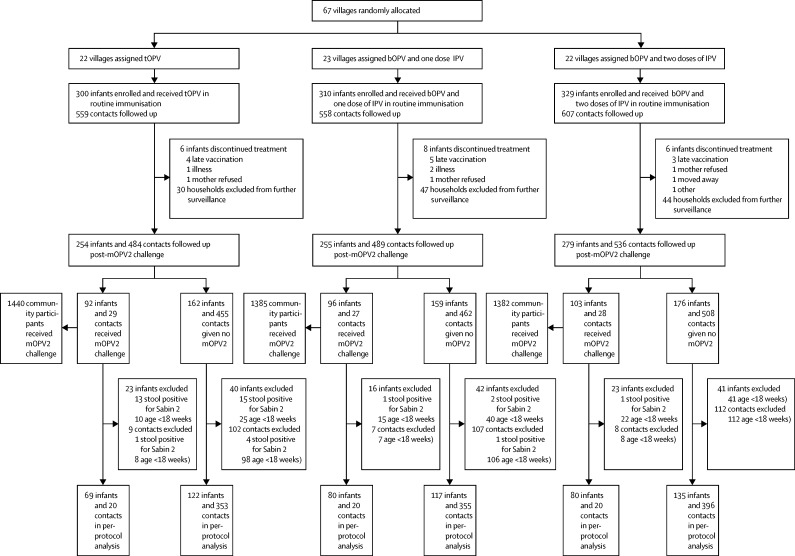
Figure 1.
Trial profile
bOPV=bivalent types 1 and 3 oral polio vaccine. IPV=inactivated polio vaccine. mOPV2=monovalent type 2 oral polio vaccine. tOPV=trivalent oral polio vaccine. Infants included in the per-protocol analysis were all children (and their household contacts) enrolled on or before Nov 1, 2015, who reached age 18 weeks by the date of the mOPV2 campaign (Jan 24, 2016) and who were not shedding Sabin 2 virus before the campaign. After the mOPV2 campaign, 788 children and their associated contacts were randomly selected from the enrolled cohort for intensive stool sampling.