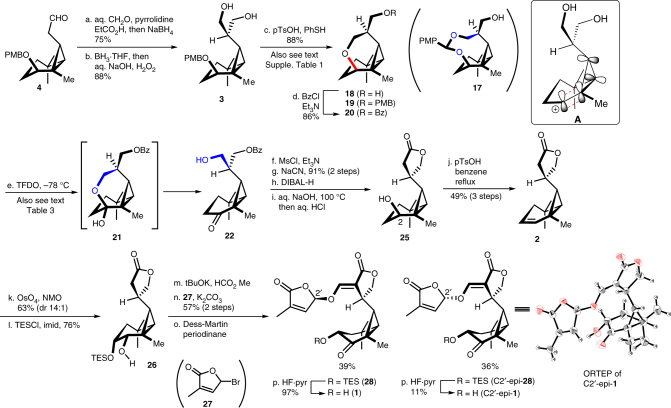
Fig. 5.
Total synthesis of avenaol (1). Conditions: a aq. CH2O, pyrrolidine, EtCO2H, iPrOH, 45 °C, then NaBH4, MeOH, 0 °C, 75%; b BH3·THF, then aq. NaOH, aq. H2O2, 88%; c p-toluenesulfonic acid, PhSH, CH2Cl2, 88%; d BzCl, Et3N, DMAP, CH2Cl2, 86%; e TFDO, CH2Cl2, −78 °C, f mesyl chloride, Et3N, CH2Cl2; g NaCN, 15-crown-5, DMSO, 91% (two steps); h diisobutylaluminium hydride, THF, −20 °C; i aq. NaOH, 100 °C, then 1 M aq. HCl; j p-toluenesulfonic acid, benzene, reflux, 49% (three steps); k OsO4, 4-methylmorpholine N-oxide, acetone–tBuOH–H2O, 63% (dr 14:1); l TESCl, imidazole, DMF, 76%; m tBuOK, HCO2Me, THF; n 5-bromo-3-methylfuran-2-one, K2CO3, 1-methyl-2-pyrrolidinone, 57% (two steps), dr 1:1; o Dess–Martin periodinane, CH2Cl2–pyr, 39% (for 28) and 36% for (C2′-epi-28); p HF·pyr, THF, 97% (for 1), 11% (for C2′ epi-1). Bz Benzoyl, TES triethylsilyl, TFDO methyl(trifluromethyl)dioxirane