Table 2.
Formation of all-cis-substituted cyclopropane
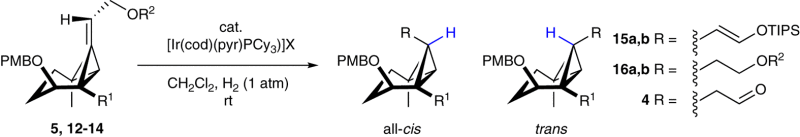
| |||||
|---|---|---|---|---|---|
| Entry | Substrate | R1 | R2 | X | Yield (all-cis:trans)a,b |
| 1 | 12 | CN | TIPS | PF6 | No reaction |
| 2 | 13 | CH2OH | TIPS | PF6 | 15a: 92% (all-cis only) |
| 3 | 14 | Me | TIPS | PF6 | 15b: 6% (2.3:1)c |
| 4 | 14 | Me | TIPS | BArF | 15b: 17% (trans only) |
| 16a: 75% (trans only) | |||||
| 5 | 5 | Me | H | PF6 | 4: 61% (2.7:1) |
| 16b: 5% (2.7:1) | |||||
| 6 | 5 | Me | H | BArF | 4: 68% (10:1) |
| 16b: <5% (2.6:1) | |||||
BArF, (3,5-bisCF3C6H3)4B−, cod, cyclooctadiene, pyr, pyridine, Cy, cyclohexyl
aIsolated yield
bThe ratio was estimated using 1H NMR spectroscopy
cStarting material 14 was recovered (64%)
