Abstract
Injectable hydrogels have gained prominence in the field of tissue engineering for minimally invasive delivery of cells for tissue repair and in the filling of irregular defects. However, many injectable hydrogels exhibit long gelation times or are not stable for long periods after injection. To address these concerns, we used thermosensitive poly(N-vinylcaprolactam) (PNVCL) hydrogels due to their cytocompatibility and fast response to temperature stimuli. Changes in the PNVCL molecular weight and concentration enabled the development of hydrogels with tunable mechanical properties and fast gelation times (<60 s when the temperature was raised from room temperature to physiologic temperature). Chondrocytes (CHs) and mesenchymal stem cells were encapsulated in PNVCL hydrogels and exhibited high viability (∼90%), as monitored by Live/Dead staining and Alamar Blue assays. Three-dimensional constructs of CH-laden PNVCL hydrogels supported cartilage-specific extracellular matrix production both in vitro and after subcutaneous injection in nude rats for up to 8 weeks. Moreover, biochemical analyses of constructs demonstrated a time-dependent increase in glycosaminoglycans (GAGs) and collagen, which were significantly augmented in the implants cultured in vivo. Histological analyses also demonstrated regular distribution of synthesized cartilage components, including abundant GAGs and type II collagen. The findings from this study demonstrate thermosensitive PNVCL as a candidate injectable biomaterial to deliver cells for cartilage tissue engineering.
Keywords: : cartilage, hydrogels, injectable
Introduction
Cartilage tissue engineering is needed due to the limited ability of native cartilage to self-repair and regenerate after damage. This necessitates new technologies and approaches to repair damage to articular cartilage, typically caused by traumatic injury and osteoarthritis that is increasingly common, especially in an aging population.1–3 Typical clinical treatments for articular cartilage repair involve microfracture, autologous chondrocyte (CH) implantation, osteochondral allograft transplantation, and scaffold base techniques.4 Although these therapies have reduced pain and delayed cartilage degeneration, they have limitations such as the need for complicated surgical procedures, risk of infections, poor integration with the surrounding tissue, and formation of fibrocartilage that has inferior mechanical and chemical properties compared to hyaline cartilage.1,2,4–6
To overcome these limitations, cell-based constructs have emerged that integrate CHs, biochemical signals, and three-dimensional (3D) scaffolds to create an engineered cartilage tissue.7 These constructs are usually composed of synthetic or natural hydrogels that can encapsulate cells, be modified to have specific mechanical properties, exhibit controlled degradation, and possess a permeable matrix for the diffusion of soluble factors, all of which can enable or promote neocartilage formation.7–11 However, preformed scaffolds demand surgical procedures for implantation, increasing the risk of infection and potentially poor integration to the defect site.5,12 Alternatively, injectable hydrogels overcome many of these concerns, since they are formed during or after the injection process.13 In this way, they can be implanted in a minimally invasive manner, provide better defect margin adaptation, and can incorporate cells, therapeutic drugs, and growth factors, which will stimulate the formation of extracellular matrix (ECM) and the repair of cartilage tissue.2,5,12
Injectable hydrogels are formed due to the in situ transition from a liquid phase to a gel state through the formation of a 3D polymeric network as a result of chemical bonds or physical interactions between polymer chains.14 Chemically cross-linked hydrogels can be formed by reactions such as radical polymerizations,9 click chemistry,15 Michael-type addition reactions,16 and dynamic covalent chemistry,17 forming in most cases irreversible cross-links.13 Alternatively, hydrogels can be formed by physical interactions as result of external stimuli, such as heat and pH,18,19 or intermolecular assemblies, such as guest–host bonds,20,21 resulting in networks with reversible bonds.13 Particularly, thermosensitive hydrogels undergo a sol–gel transition, based on hydration–dehydration processes according to temperature changes.22,23 Such hydrogels present a lower critical solution temperature (LCST), which is the temperature where the sol–gel transition occurs.24 Specifically, the thermosensitive polymer is dispersed in an aqueous solution, where the polymer chains swell in a random coil conformation due to hydrophilic interactions with water molecules; however, when the system is heated past the LCST, the polymer chains aggregate and collapse due to predominant hydrophobic interactions between polymer chains.18
Some thermosensitive polymers, for instance, poly(N-isopropylacrylamide) (PNIPAm) and poly(N-vinylcaprolactam) (PNVCL), have LCST values similar to physiological temperatures. Therefore, these materials can transition from a liquid to a gel and form an in-situ hydrogel when injected into the body, without additional chemical reactions, cross-linkers, or environmental treatments.13,24 Although both of these polymers have similar thermoresponsive behaviors, PNVCL does not produce toxic compounds when degraded and has demonstrated improved cell viability compared to PNIPAm.22,23,25,26 PNVCL assemblies degrade through the dissociation of the polymers over time, which leads to material erosion. With these characteristics and general consideration of biocompatibility, PNVCL is a promising biomaterial for biomedical applications and has gained attention, especially for therapeutic delivery and entrapment of enzymes.27–29 However, there has been little work toward the use of PNVCL in tissue engineering, especially when dealing with the investigation of cell–material interactions and tissue formation in vitro and in vivo.30–32
In the present work, we report the development and application of an injectable PNVCL hydrogel for cartilage tissue engineering. PNVCL hydrogels were synthesized with two different polymer molecular weights (MWs) and with sol–gel transition at physiological temperature. The mechanical properties against temperature and time were investigated by rheological analysis and tailored through PNVCL MW and concentration. Furthermore, the cytocompatibility and formation of tissue both in vitro and in vivo were assessed with encapsulated bovine CHs and human mesenchymal stem cells (MSCs). This work is an important step toward the development of noninvasive material-based approaches for cartilage repair.
Materials and Methods
Synthesis of PNVCL
PNVCL was synthesized through modification of a procedure by Medeiros et al.33 PNVCL with lower (PNVCL-L, Mn = 12.9 kDa, polydispersity index [PDI] = 5) or higher (PNVCL-H, Mn = 142.7 kDa, PDI = 5) MWs were synthesized by initially adding 15% or 5% w/w of N-vinylcaprolactam monomer (≥98%; Sigma-Aldrich) in the solvents dimethyl sulfoxide (DMSO, anhydrous, ≥99.9%; Sigma-Aldrich) and distilled water, respectively. Then, 2% w/w of 2,2′-azobis(2-methylpropionitrile) (AIBN, 98%; Sigma-Aldrich), previously recrystallized in methanol, was added to the reaction system at 70°C under nitrogen atmosphere. Within 4 h the polymerization was already completed and the PNVCL was purified by dialysis (MW cutoff 3.5 kDa) against deionized water during 4 days with repeated changes of water. PNVCL was then lyophilized and stored at 4°C before use.
Characterization of PNVCL
PNVCL-L and PNVCL-H molecular structures were analyzed by 1H NMR (DMX 360 MHz; Bruker, Billerica, MA). Chemical shifts (δ) are reported in ppm relative to D2O (δ = 4.81 ppm). Four hydrogen resonances were found in PNVCL-L and PNVCL-H spectra. They are assigned to: methylene groups from caprolactam ring and backbone (6H, 2H, δ = 1.8 ppm), close to C = O (2H, δ = 2.4 ppm), and close to N (2H, δ = 3.4 ppm) and − NCH − (1H, δ = 4.4 ppm). Ultraviolet (UV)-vis spectroscopy (MultiSpec-1501 UV-vis Spectrophotometer Shimadzu) coupled with a Peltier control device (TCC-Controller Shimadzu 240) was applied to determine the LCST values of both polymers. For this, the transmittance of a diluted aqueous polymer solution (1% w/v) was measured at a wavelength of 500 nm from 25°C to 35°C. The temperature was allowed to stabilize before the measurement of the transmittance.
Rheological analysis was performed on an AR2000 rheometer (TA Instruments), coupled with a Peltier control device (20 mm diameter cone and plate geometry, 59 min 42 s cone angle, gap of 27 μm). Storage (G′) and loss (G″) moduli from PNVCL-L (10–30% w/v in phosphate-buffered saline [PBS]) and PNVCL-H (5–20% w/v in PBS) were evaluated at the temperature range of 25–37°C, at 3°C/min, 0.5% strain, and 1 Hz. The time to reach elevated mechanical properties at 37°C was investigated by time sweep (300 s, 0.5% strain, 1 Hz) of intermediate concentrations of polymers—PNVCL-L (20% w/v) and PNVCL-H (10% w/v).
Cell isolation and expansion
Bovine CHs were isolated from juvenile bovine joints (Research 87, Boylston, MA), and maintained and expanded through passage 1 in basal medium, which consists of high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% v/v fetal bovine serum (FBS; Gibco-Invitrogen) and 1% v/v penicillin/streptomycin/fungizone (PSF; Gibco-Invitrogen).3,34 Human MSCs (Lonza) were maintained and expanded to passage 3 in growth media, which consists of minimum essential medium α supplemented with 16.7% w/v FBS (Gibco-Invitrogen) and 1% penicillin/streptomycin (Gibco-Invitrogen).
Cytotoxicity studies
Cytotoxicity of PNVCL-L and PNVCL-H was assessed by an Alamar Blue (AB) assay to measure viability of CHs and MSCs. Initially, CHs and MSCs were suspended in basal and growth media, respectively, seeded in a 96-well plate at a density of 1 × 104 cells per well, and incubated at 37°C, 5% CO2 for 24 h. Then, the media (total volume of 100 μL) was replaced with the appropriate polymers resuspended in media at a series of concentrations from 0.001 to 1 mg/mL and incubated for 24 h. Subsequently, media containing polymer was removed, and the cells were washed with PBS thrice at room temperature before being replaced by 100 μL per well of AB solution (10% w/w of alamarBlue® in serum-free basal medium). After incubation at 37°C for 4 h, the absorbance of each sample (n = 4) was measured in a microplate reader (excitation wavelength = 540 nm, emission wavelength = 610 nm). Cell viability (%) was calculated according to the absorbance values measured and normalized to the absorbance of control wells, in which seeded cells were incubated in the presence of media alone.
Three-dimensional cell viability and in vitro studies
CHs and MSCs were resuspended at a concentration of 20 million cells/mL in PNVCL-L (20% w/v) and PNVCL-H (10% w/v). Then, 50 μL of these suspensions were injected using hypodermic needles of various gauges (18G, 21G, 27G) into custom polydimethylsiloxane circular molds adhered to 12 mm plasma-treated glass coverslips and placed inside a 24-well plate. They were then incubated at 37°C for gelation, and 2 mL of basal or growth media was added. On the day of injection (day 0) and after 3 days (day 3), cell viability in the encapsulated materials was assessed by a Live/Dead Assay Kit (Molecular Probes) with the use of confocal microscopy (TCS SP5; Leica) to image throughout the construct volume. Live cells, stained green with calcein-AM, and dead cells, stained red with ethidium homodimer, were quantified. In vitro studies to investigate neocartilage formation were performed with CH-laden PNVCL-L and PNVCL-H cultured in the presence of 2 mL of chondrogenic medium (high-glucose DMEM, 1% v/v Corning® ITS Premix Universal Culture Supplement, 50 mg/mL l-proline, 0.1 mM dexamethasone, 0.9 mM sodium pyruvate, 50 mg/mL ascorbate, and 1% v/v of PSF),9 which was replaced thrice a week over 4 and 8 weeks.
Subcutaneous injection in nude rats
CH-laden and acellular hydrogels of PNVCL-L (20% w/v) and PNVCL-H (10% w/v) at 20 million cells/mL were prepared as previously described and injected with a hypodermic needle (21G) subcutaneously in male athymic nude rats (aged 9–10 weeks), under inhalational anesthesia (2% vaporized isoflurane in the supply gas [oxygen]), and using standard aseptic techniques. A total of 10 subcutaneous injections per timepoint, 200 μL per injection, were administered for each CH-laden hydrogel (n = 8), and 2 injections of acellular samples (n = 2) were used as controls. Samples were explanted at 4 and 8 weeks. This in vivo study was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania and the Philadelphia VA Medical Center.
Biochemical and histological analyses
At 4 and 8 weeks, the in vitro and in vivo CH-laden hydrogels (n = 4) were weighed, lyophilized, and then digested in 0.75 mg/mL of papain (Sigma) at 60°C for 48 h. The glycosaminoglycan (GAG) content in these samples was determined using the dimethylmethylene blue (DMMB) assay (Sigma-Aldrich), using a standard curve of bovine chondroitin 4-sulfate.9,35 Collagen content was also determined using the orthohydroxyproline (OHP) assay, using a trans-4-hydroxy-l-proline (Sigma-Aldrich) standard curve, and quantified according to the known ratio 1:7.14 of OHP:collagen.34,36 Acellular constructs were also evaluated in both assays as control groups, showing no interference in the data obtained.
CH-laden and acellular implants removed from the rats after 4 and 8 weeks were processed for histology in paraffin (n = 3). Sections of 7 μm of thickness were stained with Alcian blue (pH 2.5) for proteoglycans and immunostained for collagen type I and II (Col I and Col II, respectively). Sections were imaged by bright-field microscopy (Olympus BX51), and quantification of the mean staining intensity of implants was measured using ImageJ, by converting the images to 8-bit grayscale.
Statistical analysis
Statistical analysis was performed using Excel software with Daniel's XL Toolbox add-in. Significance between groups (n = 4 per group) was determined by one-way analysis of variance (ANOVA) with Tukey's post hoc test (p < 0.05), and error bars represent standard error of the mean.
Results and Discussion
Synthesis and characterization of PNVCL
PNVCL, a prominent injectable and biocompatible material that undergoes a transition phase at physiological temperature, is still poorly explored as a potential hydrogel material for tissue engineering. To understand the potential of PNVCL as an injectable biomaterial for cartilage tissue engineering, two polymers (PNVCL-L and PNVCL-H) with distinct MWs (PNVCL-L, Mn,GPC = 12.9 kDa; PNVCL-H, Mn,GPC = 142.7 kDa) were synthesized by tuning synthesis parameters (Fig. 1a). PNVCL-L was obtained in a homogenous system of solvent (DMSO), monomer, and radical initiator, whereas PNVCL-H was prepared in a heterogeneous system with water and the formation of a precipitated polymer during the reaction at 70°C (above its transition phase temperature). In both systems, a pure phase of linear PNVCL was obtained after 4 h of reaction. However, the precipitation polymerization led to a higher MW, which is usually attributed to a higher ratio of the propagation to termination rate compared to a homogenous system.37 Lozinsky et al.38 also observed an increase in the MW during the synthesis of PNVCL using a mixture of water/DMSO as the solvent compared to synthesis in DMSO alone.
FIG. 1.
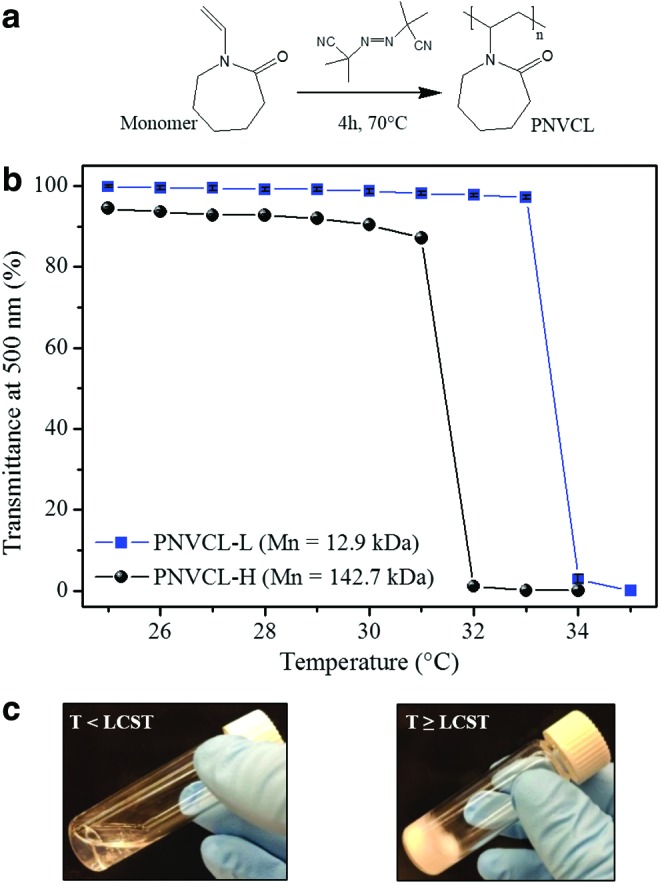
Representation of the radical polymerization of PNVCL (a). The percent transmittance (at a wavelength of 500 nm) as a variation of temperature of PNVCL suspensions (1% w/v) with different molecular weights (b). Images of the transition of a PNVCL-L suspension from transparent to opaque when below and above the LCST, respectively (c). LCST, lower critical solution temperature; PNVCL, poly(N-vinylcaprolactam); PNVCL-L, PNVCL lower. Color images available online at www.liebertpub.com/tea
The MW of PNVCL can also influence LCST values during the sol–gel transition. The reduction of the transmittance with temperature of a PNVCL aqueous suspension indicates the transition of the thermosensitive polymer from sol (transparent) to gel (opaque), as shown in Figure 1b and c; PNVCL-L and PNVCL-H presented LCST values of ∼34°C and 32°C, respectively. This reduction of the LCST with the MW can be explained according to the Flory–Huggins miscibility behavior, where PNVCL exhibits the classical phase change in water (type I) and where the critical point in the phase diagram shifts to LCST values with the increase of polymer chain length and concentration.39,40
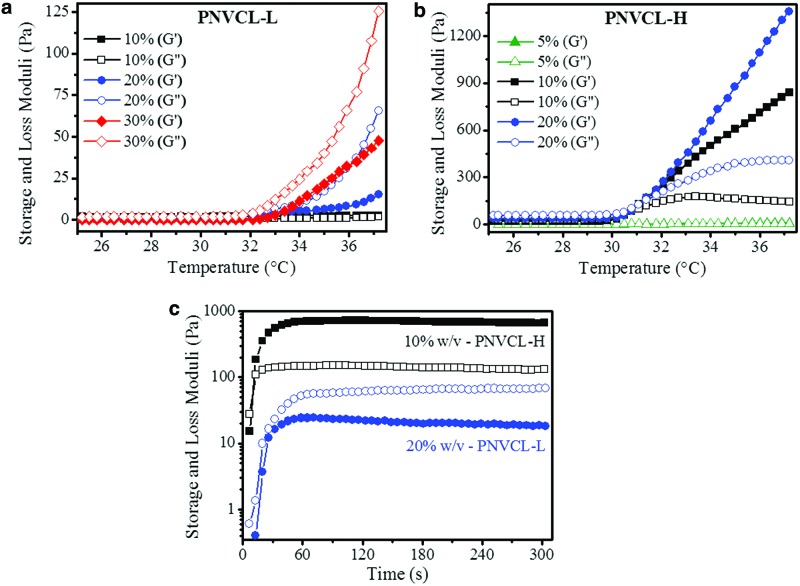
The viscoelastic properties of the hydrogels were evaluated by monitoring the storage and loss moduli (G′, G″, respectively) as a function of temperature and time (Fig. 2). Increasing the system temperature from 25°C to 37°C resulted in higher mechanical properties for all polymer suspensions; this is a result of the transition phase and the formation of the globule phase. By changing the concentration of the PNVCL-L suspension from 10% to 30% (Fig. 2a), higher mechanical properties were observed, where G″ was greater than G′ for all concentrations, a condition indicative of viscous liquid materials and where G″ reached up to 125 Pa. For PNVCL-H, the concentration varied from 5% to 20% (Fig. 2b), due to the insolubility of the polymer in PBS at higher concentrations. For all concentrations, G′ was greater than G″, a condition indicative of elastic hydrogel formation,2,41 where G′ reached up to ∼1.36 kPa. Comparing both polymer systems above the LCST, PNVCL-L cannot be considered a gel since G″ > G′. However, with the increase of the temperature and association of the PNVCL-L polymer chains with further phase separation, PNVCL-L acquires a “gel-like” behavior compared to the same material at room temperature.24,42 On the contrary, PNVCL-H reached the gel point (G′> G″) at LCST for all investigated concentrations. Different MWs between PNVCL-L and PNVCL-H explain these distinct mechanical properties, since longer chains result in more entanglement, which increases the viscosity and the elastic energy stored.43
FIG. 2.
Variation of storage (G′, solid) and loss (G″, open) moduli with temperature of PNVCL-L from 10% to 30% w/v (a) and PNVCL-H from 5% to 20% w/v (b). Increase of G′ and G″ with time for PNVCL-L (20% w/v) and PNVCL-H (10% w/v) hydrogels, starting at room temperature and increasing to 37°C, indicated by the temperature profile with time (c). PNVCL-H, PNVCL higher. Color images available online at www.liebertpub.com/tea
Two intermediate concentrations for PNVCL-L (20% w/v) and PNVCL-H (10% w/v) were then chosen (based on their mechanical properties and their solubility in medium) for further investigation in vitro and in vivo. These studies required fast gelation time and increased mechanical properties from the polymer suspensions to prevent the diffusion of the material away from the injection site and to ensure the stability of the hydrogel.44 To simulate how the mechanical properties of PNVCL hydrogels respond to the temperature stimuli imposed by physiological conditions, PNVCL-L (20% w/v) and PNVCL-H (10% w/v) maintained initially at 25°C were heated to 37°C (Fig. 2c). Both materials showed that the greatest G′ and G″ were reached in <60 s, the same time needed for the rheometer to stabilize to 37°C. This result indicates that the response of PNVCL suspensions to temperature stimuli should be almost immediate when presented into a physiological environment. Unlike injectable hydrogels formed by chemical reactions that can result in local toxicity and slow gelation,13,44 PNVCL materials exhibited tunable mechanical properties and fast response to temperature stimuli without the need for additional cross-linkers or copolymerization.
Cytocompatibility of PNVCL
Some thermosensitive hydrogels have showed cytotoxic effects, depending on their MW and concentration of polymer.25,44–46 The cytotoxicity related to the MW has been associated with hydrogels with shorter polymer chains, in particular, those that can penetrate the cell membrane, altering cell activity. In addition, in the case of polymers with higher MW, cytotoxic effects are thought to be related to the reduction of the diffusion of nutrients and oxygen to the cells, leading to cell death.46 CHs and MSCs were analyzed in the presence of PNVCL-L and PNVCL-H at a range of polymer concentrations in basal media (0.001–1 mg/mL). Cells were seeded in a 96-well plate and, subsequently, incubated for 24 h with polymer suspension, as represented in Figure 3a. CH and MSC viability was then assessed using the AB assay (Fig 3b, c, respectively). Comparing the fluorescence readouts of cells incubated in the presence of polymer with cells in basal media enabled an estimate of viability. A high cell viability of 100% was observed for PNVCL-L and PNVCL-H for all concentrations investigated, without significant differences between groups, with the exception of PNVCL-H at 1 mg/mL for CHs and 0.01 and 1 mg/mL for MSCs, as well as PNVCL-L at 1 mg/mL for MSCs. Each of these conditions showed a significant increase in the cell viability, which may be related to cell proliferation or also to a higher metabolic activity of cells in the presence of polymer.47 By observing no differences between the cell activity and the presence of PNVCL-H or PNVCL-L in several cases, however, we determined that the previously suggested effects of low versus high MW on cell viability were not observed with these polymers and that cell viability was maintained in the presence of either polymer.
FIG. 3.
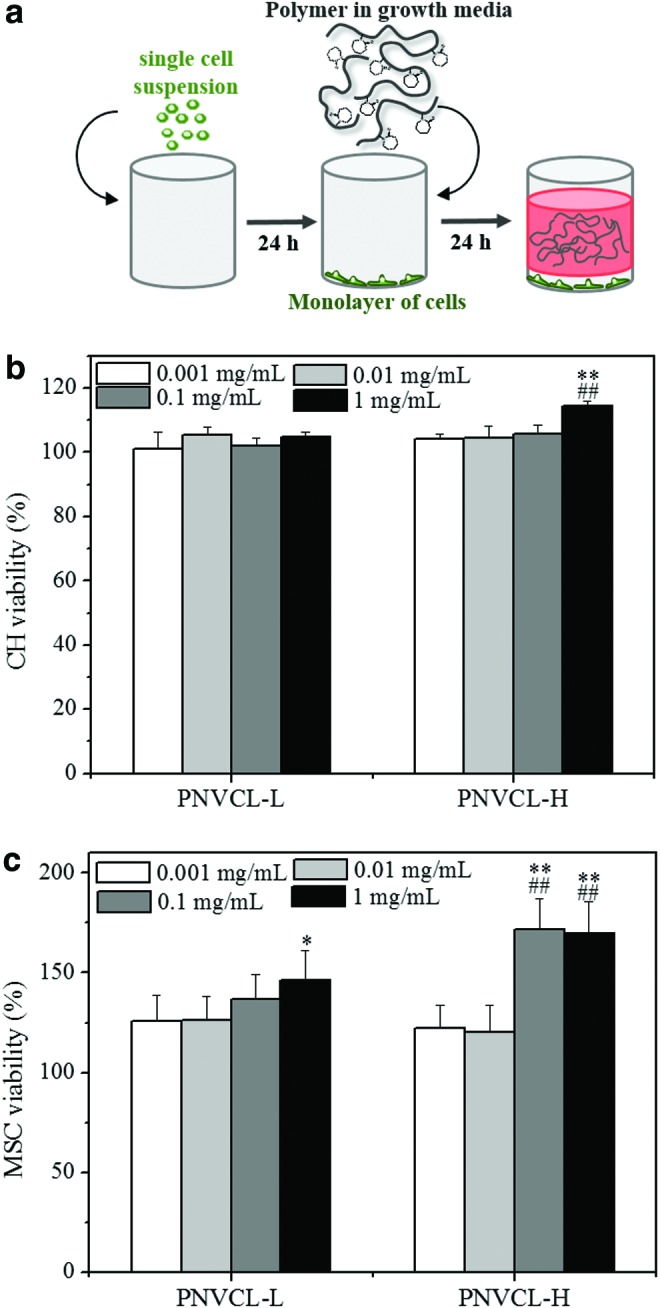
Schematic representation of the 2D system used for cytotoxicity studies. A monolayer of CHs was formed on the bottom of the well and then incubated with polymer suspensions in growth media (a). CH (b) or MSC (c) viability was assessed by Alamar Blue assay after 1 day for PNVCL-L and PNVCL-H at different polymer concentrations (0.001–1 mg/mL). *##**Indicate statistical significance (p < 0.05 and p < 0.01, respectively) versus corresponding hydrogel with concentration of 0.001 mg/mL. ##Indicates statistical significance (p < 0.01) versus the cell viability of PNVCL-L with the same polymer concentration. CH, chondrocyte; MSC, mesenchymal stem cell. Color images available online at www.liebertpub.com/tea
Three-dimensional cell viability and in vitro studies
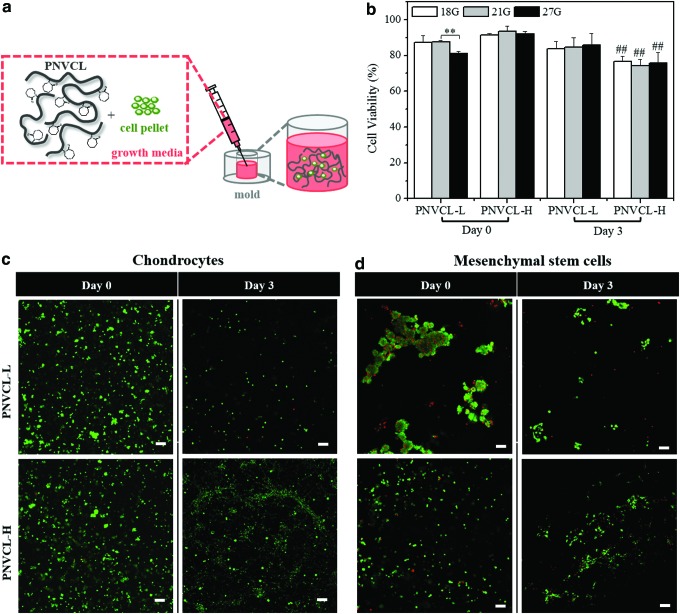
To investigate cell behavior in 3D scaffolds composed of these materials, CHs and MSCs were encapsulated in PNVCL-L (20% w/v) and PNVCL-H (10% w/v) by resuspending cell pellets in the hydrogel suspensions as shown in Figure 4a. These suspensions were injected into molds using three different hypodermic needle gauges and incubated at 37°C. Cell viability was determined using a Live–Dead Cell Staining Kit after 0 (after injection into molds) and 3 days of culture in chondrogenic media (Fig. 4b). The encapsulation of cells in thermosensitive polymers is dictated according to the temperature. At physiological conditions above the LCST, polymer hydrophobic interactions stabilize the gel and the resulting cell distribution.48 However, if the system is brought to temperatures below LCST, PNVCL polymer chains will swell with predominant hydrophilic interactions, which may disrupt cell distributions at early times before matrix is produced.
FIG. 4.
Schematic representation of the 3D system for cell encapsulation, injection in a polydimethylsiloxane mold, and incubation at 37°C (a). CH viability on the day of preparation (day 0) and after 3 days (day 3) of encapsulation in PNVCL-L (20% w/v) and PNVCL-H (10% w/v) injected with three needles of size (18G, 21G, and 27G) (b). Live/dead imaging (live cells-green; dead cells-red) of CH-laden (c) and MSC-laden (d) hydrogels injected with a needle of 21G, at days 0 and 3 (scale bars, 100 μm). **Indicates significance (p < 0.01) between groups 21G and 27G for PNVCL-L. ##Indicates significance (p < 0.01) versus the cell viability of PNVCL-H at day 0 for each gauge size. 3D, three-dimensional. Color images available online at www.liebertpub.com/tea
For PNVCL-L, CH viability remained >80% up to day 3, without significant differences among the days and needle gauge used. Only a slight reduction was observed for the cell viability at day 0 and with 27G needle use, most likely due to the mechanical shear on cells during the injection through a smaller needle.41 In contrast, CH-laden PNVCL-H hydrogels had viability >90% at day 0 and ∼75% at day 3. This reduction may be associated with the tendency of thermosensitive polymers with higher MW to form a denser hydrogel, which can reduce the diffusion of nutrients and oxygen.46,49 In addition, no significant differences in viability were observed for different needle sizes. In this way, regardless of the MW and mechanical properties of PNVCL-L and PNVCL-H, these hydrogels effectively delivered encapsulated viable cells independent of the needle size used.
Figure 4c and d shows live/dead stained images of CHs and MSCs encapsulated in the hydrogels, respectively, after injection with a 21G needle. Viable CHs were homogenously distributed in the 3D system, while MSC-laden PNVCL-L gels exhibited some cell agglomerations, making them difficult to quantify. These differences in cell distribution in the PNVCL-L matrix suggest the influence of the cell–scaffold interaction on morphology.50 Differences in contractile behavior of cell types also influence their interaction with external mechanical forces imposed by polymeric substrates with specific stiffnesses.51,52
At day 3, the number of cells was reduced compared to day 0 for both systems of encapsulated hydrogels. This could be an unavoidable consequence of media changes, where hydrogels were temporarily exposed to room temperature conditions. Given their reversible sol–gel transition with temperature, this could possibly lead to the release of cells from the materials. This reduction in cell number after 3 days of cell culture was less pronounced for the PNVCL-H, where a higher MW resulted in greater entanglements of polymer chains, forming a dense hydrogel that could better retain the encapsulated cells.53 In this context, encapsulated PNVCL-H hydrogels showed a better cell retention during in vitro studies than PNVCL-L.
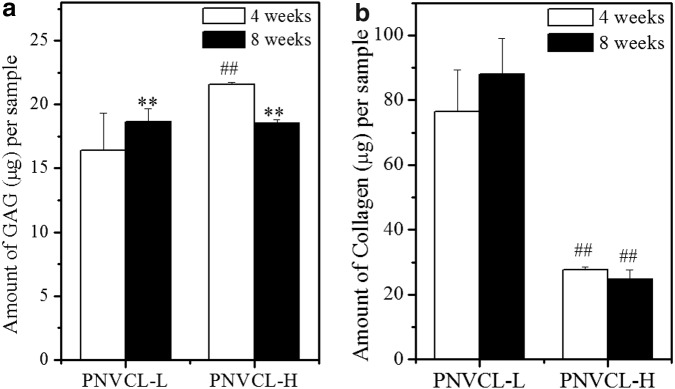
Scaffolds intended for cartilage repair/regeneration should maintain the phenotype of CHs and enable cellular function in 3D.10,54 CH-laden hydrogels in chondrogenic media were cultured in vitro for 4 and 8 weeks, and the GAG and collagen contents per construct at each timepoint were analyzed by DMMB and OHP assays, respectively, as shown in Figure 5. MSC cultures were not investigated further at this point, due to the aggregation observed. Similar GAG content was observed for both hydrogels after 4 and 8 weeks with only minor differences, with up to 21 μg GAG per sample as depicted in Figure 5a. PNVCL-H had a higher amount of GAG than PNVCL-L after 4 weeks, and similar contents were observed after 8 weeks for both hydrogels. Increasing the culture time led to higher amounts of GAG content in PNVCL-L constructs and reduced content in PNVCL-H gels. The similar amounts of GAG produced and retained in both materials at specific timepoints may be related to the release of cells to the media that was periodically replaced, as described above.
FIG. 5.
Amount of GAG (a) and total collagen (b) per sample (V = 50 μL) of CH-laden PNVCL-L (20% w/v) and PNVCL-H (10% w/v) hydrogels after 4 and 8 weeks of in vitro culture. **Indicates significance (p < 0.01) between 4 and 8 weeks for the same polymer. ##Indicates significance (p < 0.01) between PNVCL-L and PNVCL-H at 4 or 8 weeks. GAG, glycosaminoglycan.
In contrast, the collagen content produced by CHs was dependent on the encapsulated matrix. As shown in Figure 5b, CH-laden PNVCL-L gels yielded up to 3.5-fold more collagen per construct than PNVCL-H gels after both 4 and 8 weeks. Although a slight increase of collagen for PNVCL-L was observed at 8 weeks compared to 4 weeks, no statistical difference was observed between timepoints for both hydrogels. These results suggest that PNVCL hydrogels with different MWs support cartilage matrix production and maintain the phenotype of CHs in 3D culture in vitro. The ability of PNVCL-L to support more collagen production than PNVCL-H may be related to differences in their mechanical properties. Soft hydrogels have been shown to improve matrix production by CHs, induced by better nutrient diffusion, distribution of newly synthesized cartilage matrix, and differences in cell–cell interactions and distribution.9,55
Biochemical and histological analyses of hydrogels subcutaneously injected into nude rats
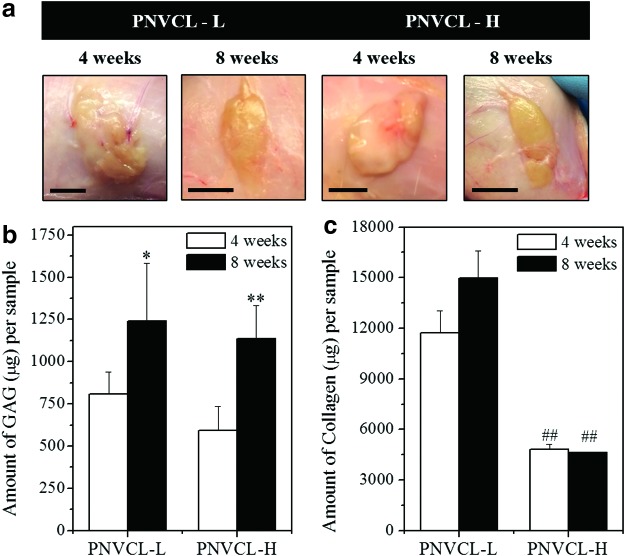
To investigate the stability of thermosensitive PNVCL and its ability to support neocartilage formation in vivo, CH-laden hydrogels were subcutaneously injected into nude rats. At subsequent time intervals, implants were removed from the subcutaneous space and analyzed as shown in Figure 6. Implants were stable throughout the 8-week study and remained localized at the injection site, with some formation of a fibrous capsule. Figure 6a also showed that implants of PNVCL-L were more spread and flatter than PNVCL-H implants, which presented a more compact shape, likely due to variations in material viscosity.
FIG. 6.
Images of the subcutaneous implants after 4 and 8 weeks (scale bars, 1 cm) (a). Amount of GAG (b) and collagen (c) per sample (V = 200 μL) of CH-laden PNVCL-L (20% w/v) and PNVCL-H (10% w/v) hydrogels after 4 and 8 weeks of in vivo culture. *##**Indicate significance (p < 0.05 and p < 0.01, respectively) between 4 and 8 weeks for the same polymer. ##Indicates significance (p < 0.01) between PNVCL-L and PNVCL-H at 4 or 8 weeks. Color images available online at www.liebertpub.com/tea
As shown in Figure 6b, no significant differences in GAG content per construct were observed for PNVCL-L and PNVCL-H at 4 and 8 weeks. Although similar behavior was observed during in vitro studies, a much greater production of GAG (up to 1245 μg per sample) was attained in vivo, especially in the case of PNVCL-L injections, which generally accumulated greater GAG than PNVCL-H implants. In addition, a time-dependent increase of GAG content per construct was observed with both PNVCL-L and PNVCL-H implants, exhibiting an increase of 1.5 and 1.9-fold, respectively, after 8 weeks compared to 4 weeks. Better results in vivo than in vitro can be attributed to the lack of destabilization of the system due to fluctuations in temperature during handling for in vitro culture, which can interfere in the retention of cells and synthesized cartilage components in the hydrogels. Finally, Figure 6c shows the collagen content in constructs after 4 and 8 weeks, which followed the same trends as were observed in the in vitro analyses, including the ratio of collagen produced by PNVCL-L and PNVCL-H between the two timepoints. Overall, taking into account the injected volume of hydrogel used for each condition studied, significant increases of these cartilage components were observed in CH-hydrogels that were subcutaneously injected compared to those cultured in vitro.
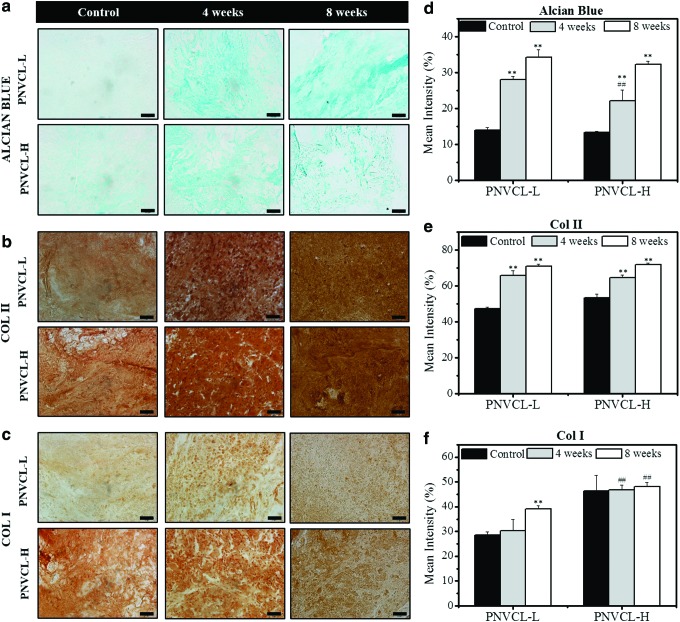
To evaluate the distribution of synthesized GAGs and collagen types I and II, implant sections were stained for these cartilage components and imaged. The intensity of staining was quantified using ImageJ. Figure 7a and d shows a time-dependent increase in Alcian blue staining compared to the control group (acellular gels after 4 weeks of implantation). PNVCL-L constructs exhibited more evenly distributed Alcian blue staining than PNVCL-H, although no statistical differences were observed between their staining intensities. Articular cartilage is comprised mainly of type II collagen and, in small or negligible amounts, type I collagen56; immunohistochemical staining against type I and II collagen was used in this study to identify the majority component that accumulated in the CH-laden hydrogels. Increases in Col II staining over time can be observed evenly distributed in both PNVCL-L and PNVCL-H constructs in Figure 7b and e compared to control groups. In contrast, Figure 7c and f shows comparable Col I staining among the control groups and all implants after 4 and 8 weeks. A slightly higher intensity was observed at 8 weeks in PNVCL-L gels, which could be attributed to the diffusion of Col I from the fibrous capsule that formed around the implant. When comparing the histology data with biochemical analysis, it is possible to note that although CH-laden PNVCL-H showed similar collagen content after 4 and 8 weeks, an intense and time-dependent increase of Col II staining was observed.
FIG. 7.
Images (a–c) and quantification (d–f) for Alcian blue (top) and immunohistochemical staining of type II collagen (Col II, middle) and type I collagen (Col I, bottom) of encapsulated CHs or acellular control (4 weeks) in PNVCL-L (20% w/v) and PNVCL-H (10% w/v) hydrogels after 4 and 8 weeks of subcutaneous implantation in rats. Scale bars for (a) are 100 μm and for (b, c) are 50 μm. *11,32,60–63**Indicate significance (p < 0.05 and p < 0.01, respectively) versus control groups (PNVCL-L or PNVCL-H). ##Indicates significance (p < 0.01) between PNVCL-L and PNVCL-H at 4 or 8 weeks. Color images available online at www.liebertpub.com/tea
Despite some studies with injectable hydrogels for cartilage tissue engineering having shown neocartilage formation,2,57–59 some characteristics such as long gelation times, sample instability, and poor matrix production can be noted.11,32,60–63 In this study, in the case of the thermosensitive polymer PNVCL, hydrogels were produced by simple methods with distinct mechanical properties and fast response to temperature stimuli. They could also encapsulate viable CHs and support neocartilage formation after in vitro culture and after in vivo subcutaneous injections in nude rats for 8 weeks.
Conclusions
In the present study, thermosensitive injectable hydrogels of PNVCL with tunable mechanical properties and fast response to temperature stimuli showed great potential for application to cartilage repair. PNVCL hydrogels with different MWs supported viable cell encapsulation of CHs and MSCs, as well as cartilage matrix production, maintaining the phenotype of CHs during 3D culture in vitro. Furthermore, subcutaneous injections in nude rats resulted in stable CH-laden hydrogel implants that produced cartilage ECM components in much greater amounts than with in vitro studies. Cell-laden PNVCL-L constructs with lower mechanical properties also showed increased ECM accumulation compared to those with PNVCL-H, possibly due to superior nutrient diffusion and more favorable mechanics for CH function. Therefore, PNVCL hydrogels show promise as scaffolds for cartilage tissue engineering.
Acknowledgments
This work was supported by São Paulo Research Foundation (FAPESP) grants 2015/07185-1 and 2013/25663-2, CEPID/CDMF (FAPESP) grant 2013/07296-2, and the National Institutes of Health (R01 EB008722) and a National Science Foundation Graduate Research Fellowship (to M.K.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Chung C., and Burdick J.A. Engineering cartilage tissue. Adv Drug Del Rev 60, 243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren K., He C., Xiao C., Li G., and Chen X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 51, 238, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Erickson I.E., Huang A.H., Sengupta S., Kestle S., Burdick J.A., and Mauck R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage 17, 1639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., and Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11, 21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivashanmugam A., Arun Kumar R., Vishnu Priya M., Nair S.V., and Jayakumar R. An overview of injectable polymeric hydrogels for tissue engineering. Eur Polym J 72, 543, 2015 [Google Scholar]
- 6.Temenoff J.S., and Mikos A.G. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 21, 431, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Huang B.J., Hu J.C., and Athanasiou K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian L., Guvendiren M., Mauck R.L., and Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian L., Hou C., Tous E., Rai R., Mauck R.L., and Burdick J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 34, 413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim I.L., Mauck R.L., and Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 32, 8771, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park K.M., Lee S.Y., Joung Y.K., Na J.S., Lee M.C., and Park K.D. Thermosensitive chitosan–pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater 5, 1956, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Jin R., Moreira Teixeira L.S., Krouwels A., Dijkstra P.J., van Blitterswijk C.A., Karperien M., and Feijen J. Synthesis and characterization of hyaluronic acid–poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater 6, 1968, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Q.V., Huynh D.P., Park J.H., and Lee D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: a review. Eur Polym J 72, 602, 2015 [Google Scholar]
- 14.Yang J.-A., Yeom J., Hwang B.W., Hoffman A.S., and Hahn S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci 39, 1973, 2014 [Google Scholar]
- 15.Desai R.M., Koshy S.T., Hilderbrand S.A., Mooney D.J., and Joshi N.S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 50, 30, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Guvendiren M., and Burdick J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 3, 792, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Yang T., Ji R., Deng X.-X., Du F.-S., and Li Z.-C. Glucose-responsive hydrogels based on dynamic covalent chemistry and inclusion complexation. Soft Matter 10, 2671, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Klouda L. Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur J Pharm Biopharm 97, Part B, 338, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Liu X.J., Li H.Q., Zhang B.Y., Wang Y.J., Ren X.Y., Guan S., and Hui Gao G. Highly stretchable and tough pH-sensitive hydrogels with reversible swelling and recoverable deformation. RSC Adv 6, 4850, 2016 [Google Scholar]
- 20.Rodell C.B., Kaminski A.L., and Burdick J.A. Rational design of network properties in guest–host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 14, 4125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodell C.B., Mealy J.E., and Burdick J.A. Supramolecular guest–host interactions for the preparation of biomedical materials. Bioconjug Chem 26, 2279, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Ponce-Vargas S.M., Cortez-Lemus N.A., and Licea-Claveríe A. Preparation of poly(N-vinylcaprolactam) (NVCL) and statistical copolymers of NVCL with variable cloud point temperature by using a trithiocarbonate RAFT agent. Macromol Symp 325–326, 56, 2013 [Google Scholar]
- 23.Ramos J., Imaz A., and Forcada J. Temperature-sensitive nanogels: poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym Chem 3, 852, 2012 [Google Scholar]
- 24.Klouda L., and Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm 68, 34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vihola H., Laukkanen A., Valtola L., Tenhu H., and Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 26, 3055, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Liang X., Kozlovskaya V., Chen Y., Zavgorodnya O., and Kharlampieva E. Thermosensitive multilayer hydrogels of poly(N-vinylcaprolactam) as nanothin films and shaped capsules. Chem Mater 24, 3707, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortez-Lemus N.A., and Licea-Claverie A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog Polym Sci 53, 1, 2016 [Google Scholar]
- 28.Rejinold N.S., Chennazhi K.P., Nair S.V., Tamura H., and Jayakumar R. Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr Polym 83, 776, 2011 [Google Scholar]
- 29.Markvicheva E.A., Tkachuk N.E., Kuptsova S.V., Dugina T.N., Strukova S.M., Kirssh Y.E., Zubov V.P., and Rumish L.D. Stabilization of proteases by entrapment in a new composite hydrogel. Appl Biochem Biotechnol 61, 75, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Shakya A.K., Holmdahl R., Nandakumar K.S., and Kumar A. Polymeric cryogels are biocompatible, and their biodegradation is independent of oxidative radicals. J Biomed Mater Res A 102, 3409, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A., and Kumar A. Thermoresponsive poly(N-vinylcaprolactam) cryogels: synthesis and its biophysical evaluation for tissue engineering applications. J Mater Sci Mater Med 21, 2937, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lynch B., Crawford K., Baruti O., Abdulahad A., Webster M., Puetzer J., Ryu C., Bonassar L.J., and Mendenhall J. The effect of hypoxia on thermosensitive poly(N-vinylcaprolactam) hydrogels with tunable mechanical integrity for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater 2016; DOI: 10.1002/jbm.b.33705 [DOI] [PubMed] [Google Scholar]
- 33.Medeiros S.F., Barboza J.C.S., Ré M.I., Giudici R., and Santos A.M. Solution polymerization of N-vinylcaprolactam in 1,4-dioxane. Kinetic dependence on temperature, monomer, and initiator concentrations. J Appl Polym Sci 118, 229, 2010 [Google Scholar]
- 34.Kim M., Erickson I.E., Choudhury M., Pleshko N., and Mauck R.L. Transient exposure to TGF-3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater 11, 92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farndale R.W., Buttle D.J., and Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 37.Kricheldorf H.R. Handbook of Polymer Synthesis. New York: Marcel Dekker Inc., 1991 [Google Scholar]
- 38.Lozinsky V.I., Simenel I.A., Kurskaya E.A., Kulakova V.K., Galaev I.Y., Mattiasson B., Grinberg V.Y., Grinberg N.V., and Khokhlov A.R. Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer 41, 6507, 2000 [Google Scholar]
- 39.Meeussen F., Nies E., Berghmans H., Verbrugghe S., Goethals E., and Du Prez F. Phase behaviour of poly(N-vinyl caprolactam) in water. Polymer 41, 8597, 2000 [Google Scholar]
- 40.Liu J., Debuigne A., Detrembleur C., and Jérôme C. Poly(N-vinylcaprolactam): a thermoresponsive macromolecule with promising future in biomedical field. Adv Healthc Mater 3, 1941, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Aguado B.A., Mulyasasmita W., Su J., Lampe K.J., and Heilshorn S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18, 806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu L., and Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev 37, 1473, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Sperling L.H. Introduction to Physical Polymer Science. New Jersey: John Wiley & Sons Inc., 2005 [Google Scholar]
- 44.Patenaude M., Smeets N.M.B., and Hoare T. Designing injectable, covalently cross-linked hydrogels for biomedical applications. Macromol Rapid Commun 35, 598, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Patenaude M., and Hoare T. Injectable, degradable thermoresponsive poly(N-isopropylacrylamide) hydrogels. ACS Macro Lett 1, 409, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Mellati A., Valizadeh Kiamahalleh M., Dai S., Bi J., Jin B., and Zhang H. Influence of polymer molecular weight on the in vitro cytotoxicity of poly (N-isopropylacrylamide). Mater Sci Eng C 59, 509, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Williams C.G., Malik A.N., Kim T.K., Manson P.N., and Elisseeff J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 26, 1211, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Lee B., Jiao A., Yu S., You J.B., Kim D.-H., and Im S.G. Initiated chemical vapor deposition of thermoresponsive poly(N-vinylcaprolactam) thin films for cell sheet engineering. Acta Biomater 9, 7691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar D., Lyness A., Gerges I., Lenardi C., Forsyth N.R., and Liu Y. Stem cell delivery with polymer hydrogel for treatment of intervertebral disc degeneration: from 3D culture to design of the delivery device for minimally invasive therapy. Cell Transplant 25, 2213, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Chen J.-P., and Cheng T.-H. Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol Biosci 6, 1026, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Peyton S.R., Ghajar C.M., Khatiwala C.B., and Putnam A.J. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys 47, 300, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Yourek G., Hussain M.A., and Mao J.J. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J 53, 219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakya A.K., Kumar A., and Nandakumar K.S. Adjuvant properties of a biocompatible thermo-responsive polymer of N-isopropylacrylamide in autoimmunity and arthritis. J R Soc Interface 8, 1748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elisseeff J. Injectable cartilage tissue engineering. Expert Opin Biol Ther 4, 1849, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Schuh E., Hofmann S., Stok K., Notbohm H., Müller R., and Rotter N. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J Biomed Mater Res A 100A, 38, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Roberts S., Menage J., Sandell L.J., Evans E.H., and Richardson J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 16, 398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L.-S., Du C., Toh W.S., Wan A.C.A., Gao S.J., and Kurisawa M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 35, 2207, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Yuan L., Li B., Yang J., Ni Y., Teng Y., Guo L., Fan H., Fan Y., and Zhang X. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte cehaviors. Tissue Eng Part A 22, 899, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Park K.-H., Lee D.H., and Na K. Transplantation of poly(N-isopropylacrylamide-co-vinylimidazole) hydrogel constructs composed of rabbit chondrocytes and growth factor-loaded nanoparticles for neocartilage formation. Biotechnol Lett 31, 337, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Peroglio M., Grad S., Mortisen D., Sprecher C.M., Illien-Jünger S., Alini M., and Eglin D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J 21, 839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peroglio M., Eglin D., Benneker L.M., Alini M., and Grad S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J 13, 1627, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Taguchi T., Xu L., Kobayashi H., Taniguchi A., Kataoka K., and Tanaka J. Encapsulation of chondrocytes in injectable alkali-treated collagen gels prepared using poly(ethylene glycol)-based 4-armed star polymer. Biomaterials 26, 1247, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Park H., Choi B., Hu J., and Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 9, 4779, 2013 [DOI] [PubMed] [Google Scholar]