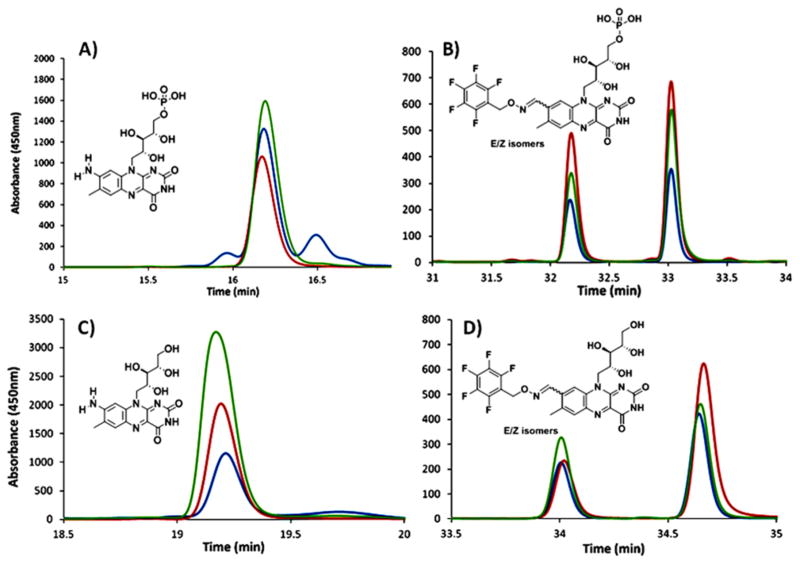
Figure 7.
Co-elution experiments with enzymatically formed formyl-FMN and AFMN and synthesized standards. (A) HPLC analysis of enzymatically formed AFMN (blue trace, enzymatically formed AFMN; red trace, AFMN standard; and green trace, co-elution of standard and enzymatic reaction mixture). (B) HPLC analysis of enzymatically formed formyl-FMN derivatized with pentafluorobenzyl hydroxylamine (blue trace, enzymatically formed formyl-FMN (absence of glutamate); red trace, formyl-FMN PFBHA oxime standard; and green trace, co-elution of standard and enzymatic reaction mixture after treatment with pentafluorobenzyl hydroxylamine). Diagrams (C and D) represent the same set of samples after phosphatase treatment showing comigration of amino-riboflavin and formyl-riboflavin PFBHA oxime with the dephosphorylated enzymatic products derivatized with PFBHA.