Fig. 1.
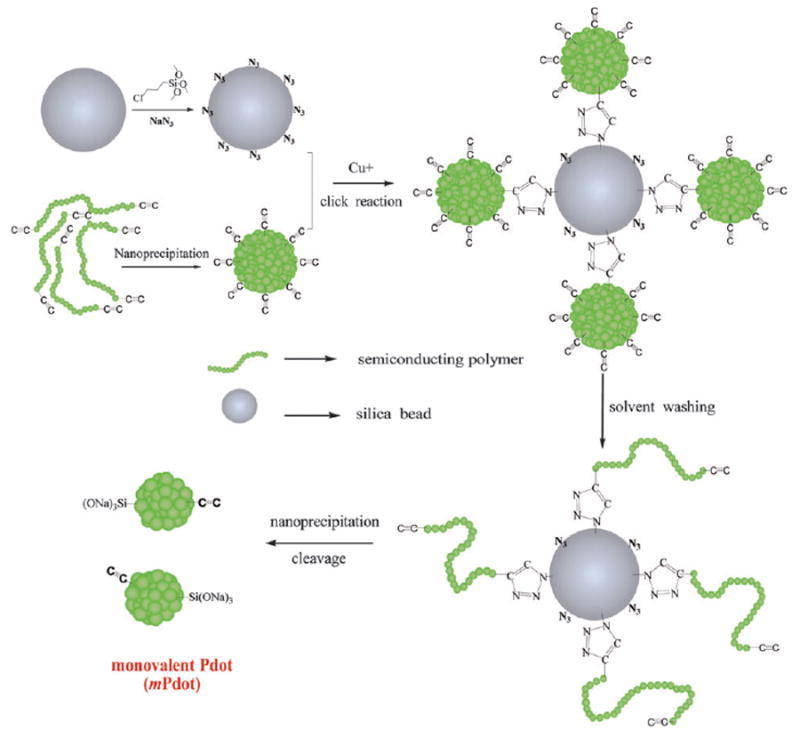
The procedure to prepare monovalent Pdots (mPdots). A silica particle with a diameter of ~200 nm was prepared and its surface modified with a layer of chloride (SiO2–Cl) via the hydrolysis and condensation of chloridetrimethoxysilane. The SiO2–Cl groups were then modified to azide to form a clickable silica nanoparticle (ESI†). Separately, regular multivalent PPV–PPA Pdots were prepared using nanoprecipitation (ESI†); these Pdots had alkyne groups so they could react with SiO2–N3 on the silica surface via click chemistry. Once the regular PPV–PPA Pdots were clicked onto the surface of the silica nanoparticle, the solvent was changed from aqueous solution to THF and the silica–polymer complex was washed with THF several times. This step removed all the polymer chains in the regular Pdot that were not covalently attached to the silica surface. The single polymer chains attached to the surface of the silica nanoparticles were then reprecipitated into small and monovalent mPdots by reintroducing the silica–polymer complex into aqueous solution from THF. Finally, the mPdots were cleaved from the silica surface and released into solution in the presence of NaOH and Triton 100. To remove NaOH and Triton 100, the mPdot solution was dialyzed overnight in water or buffer.
