Fig. 1. Development of DPI analogs.
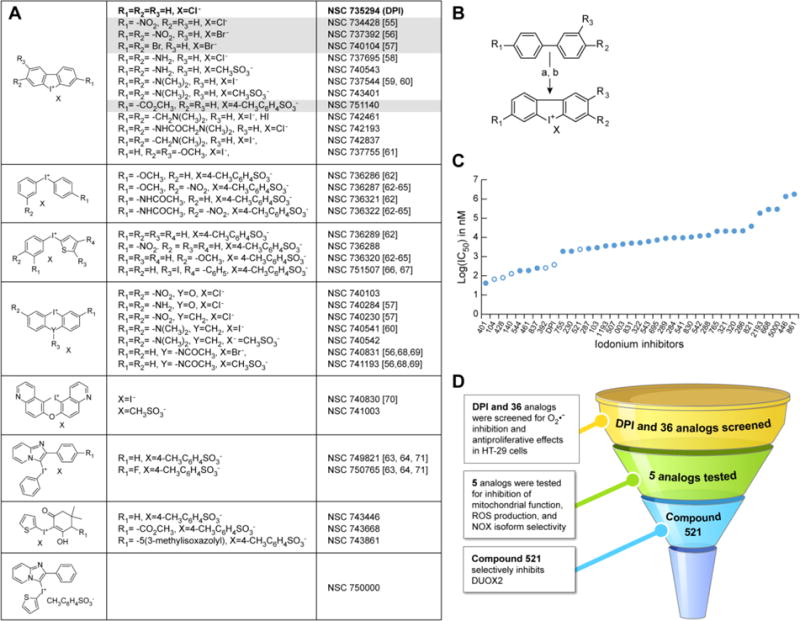
(A) Structures of DPI and 35 iodonium-class analogs. The structure for the thirty-sixth analog, compound NSC 780521 (521), is displayed in fig. 6A. DPI is shown in bold font, and the lead compounds described in the present study are highlighted in grey. (B) Synthetic pathway for the production of substituted DPI analogs. Reagents: a) I2, KIO3, H2SO4; b) KI. (C) IC50 values for iodonium compound inhibition of HT-29 cell proliferation assessed with the MTT assay at 48 h. Open circles indicate compounds described in the study. (D) Flowchart demonstrating the screening procedure for the identification of potent iodonium analogs.
