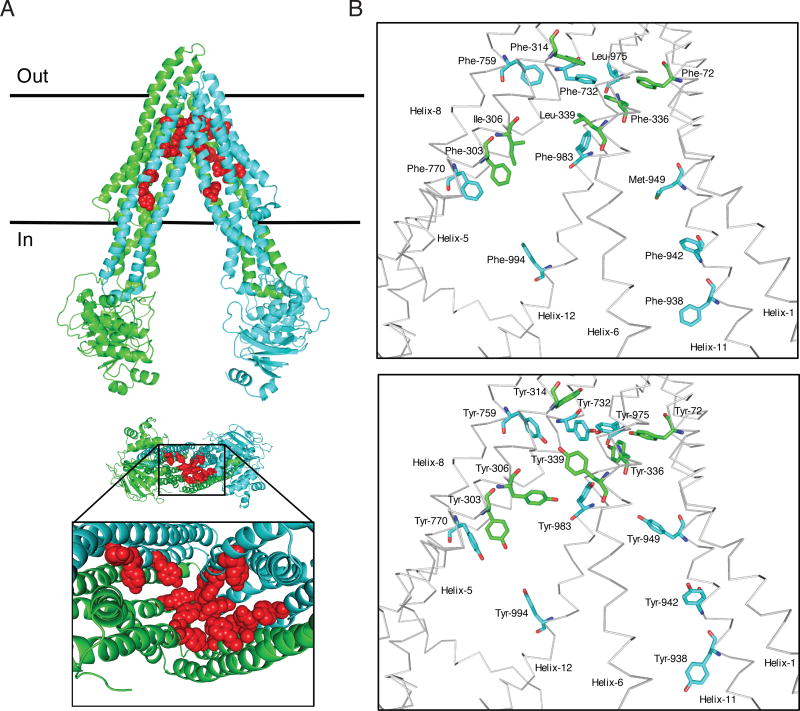
Fig. 1. Schematic presentation of the location of fifteen residues mutated in 15Y mutant P-gp.
The homology model of human P-gp was built based on the mouse P-gp crystal structure (4Q9H.pdb). Schematic presentation of WT and 15Y mutant P-gps, generated by PyMOL. (A) Side view of 15Y P-gp protein (top). The N-terminal transmembrane domain and NBD1 and C-terminal transmembrane domain and NBD2 of P-gp are highlighted in green and cyan, respectively. The fifteen residues which are replaced by tyrosine are shown in sphere format in red. The expanded bottom view depicts location of 15 tyrosine residues in the transmembrane drug-binding pocket. (B) The residues in the transmembrane region of WT protein selected for substitution and after replacement with tyrosine residues in 15Y mutant are shown in stick format at top and bottom, respectively. The protein backbone is in grey color. Mutated residues and helices are labeled. Helices 2, 3, 4, 9, and 10 were deleted for clarity.