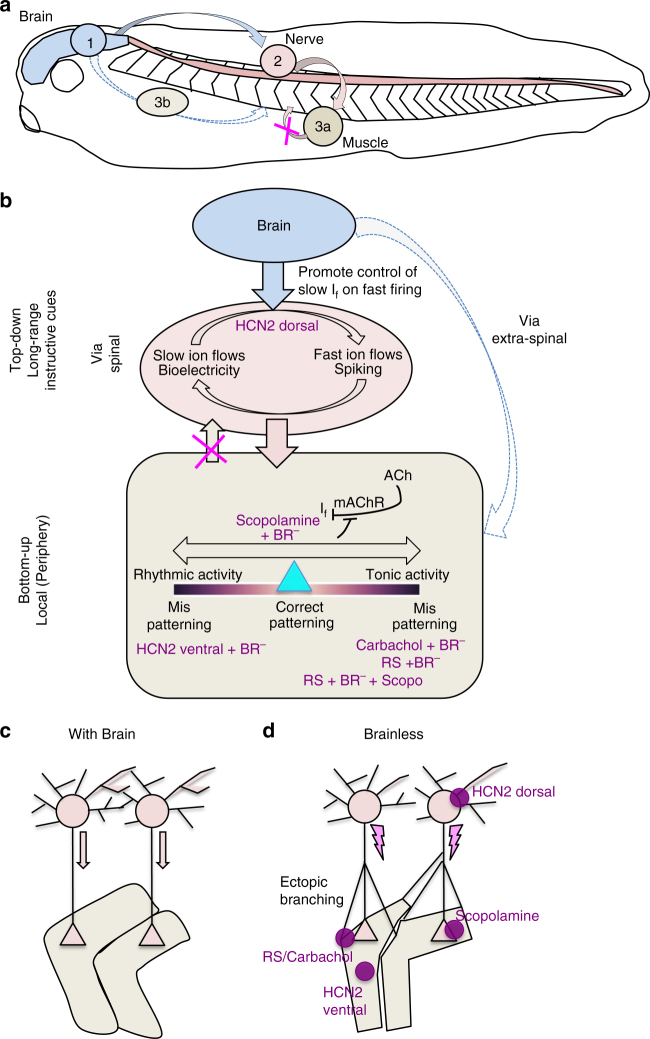
Fig. 7.
Brain signaling for muscle and nerve development and patterning a. Schematic representation drawing of a Xenopus embryo, showing the main components of our experiments: brain (blue), spinal cord-peripheral nerves (pink) and somites-muscle (brown). Brain effects on nerve patterning could occur directly (2), by using efferent spinal pathway. Brain effects on muscle patterning could occur indirectly (3a), by acting on neurons, or directly (3b), by acting on muscle. b A spinal mechanism, for coding the information about patterning and morphogenesis, could occur via direct signaling from the brain to the neurons in the spinal cord (pink circle). According to our results (different treatments are indicated with purple labels), the effects of the peripheral innervation on muscle cells can be partially explained in terms of developmental bioelectricity or changes in Vmem excitability. We hypothesize that at these stages, the brain is in part controlling the bioelectric state of peripheral tissues, and a correct balance (turquoise triangle) of brain activity (long-range instructive cues or top-down perspective) and local signals (bottom-up perspective) is necessary for correct morphogenesis. Both an excess of tonic activity (induced after carbachol or RS treatment) and an excess of slow If gradients through membrane lead to mispatterning. The extra-spinal pathway by which the brain is acting on muscles can be mimicked pharmacologically, with pharmacological agents targeting bioelectricity (i.e., scopolamine). We hypothesize that scopolamine is acting at presynaptic/synaptic level, blocking the inhibitory ACh actions (via mAChRs) on slow ion flows, and leading the Vmem to appropriate values for muscle patterning. c, d. Schematic representation of neuromuscular specificity in normal development (c, with brain) and in absence of the brain (d, BR−). Our results suggest that ectopic branching detected in the absence of a brain is not due to deficits in early pruning or target retrograde signaling. Pathfinding behavior at the onset of Xenopus development starts at the spinal cord level, as early patterned electrical gradients in SC cells is required for the correct axon guidance. The different treatments applied in our experiments (purple labels and circles) are placed on the cellular/subcellular domains where they are probably acting