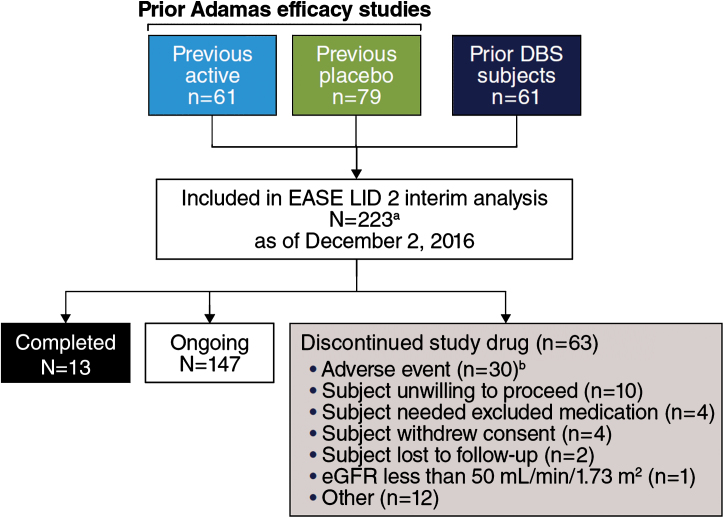
Fig.1.
Trial Profile. aStudy includes 22 patients who enrolled after a time gap following participation in double-blind studies (EASED, EASE LID, EASE LID 3) and were not summarized as a subgroup in subsequent analyses due to small sample size. bCase report form in interim data-cut for 2 additional patients reported study drug withdrawal as action taken in response to AE, while disposition record reported “other” and “ongoing.”