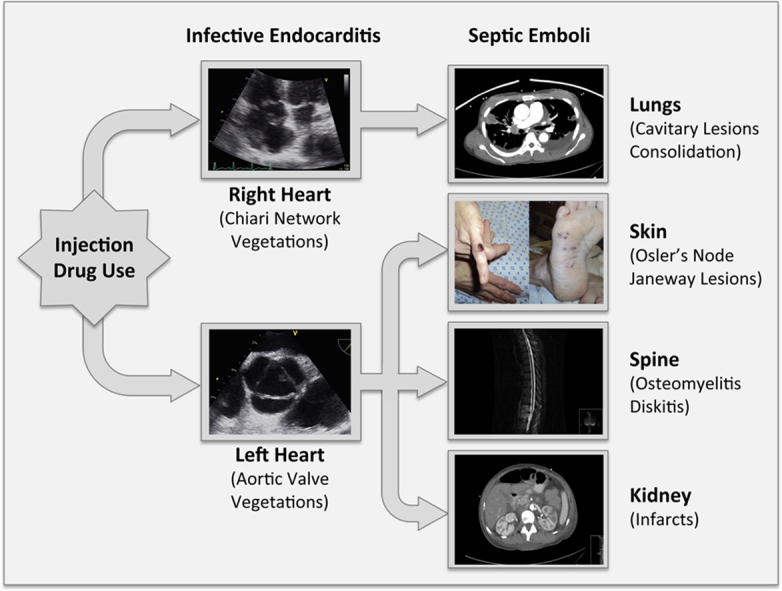
Graphical abstract
Keywords: Chiari network, Infective endocarditis, Janeway lesions, Osler's nodes, Septic emboli
Highlights
-
•
Janeway lesions and Osler's nodes were clinical clues for the diagnosis of endocarditis.
-
•
The Chiari network, a benign right atrial structure, served as a nidus of infection.
-
•
Chiari network endocarditis occurred without tricuspid valve involvement.
-
•
Bilateral endocarditis was associated with both pulmonary and systemic emboli.
-
•
Large Chiari network vegetation resolved with antibiotic therapy alone (no surgery).
Introduction
Infective endocarditis occurs in about 13% of febrile injection drug users1 and involves the right and left heart valves with equal frequency.2 Concurrent right-sided and left-sided endocarditis (bilateral endocarditis) has been reported in 9%-13% of intravenous drug users with endocarditis.2, 3, 4 Chiari network or Eustachian valve as the primary focus of right-sided endocarditis, in the absence of tricuspid valve involvement, is rare.5, 6 We present a case of bilateral infective endocarditis due to Staphylococcus aureus in an intravenous drug user with pulmonary and systemic embolization, where the primary right-sided cardiac involvement was a very large vegetation on the Chiari network rather than on the tricuspid valve.
Case Presentation
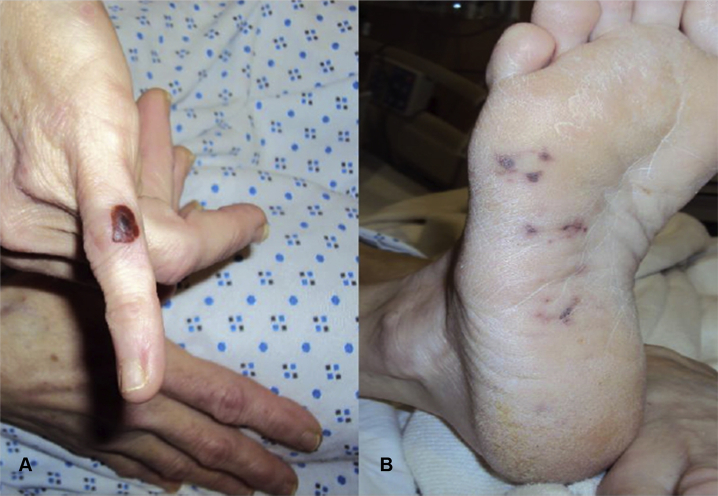
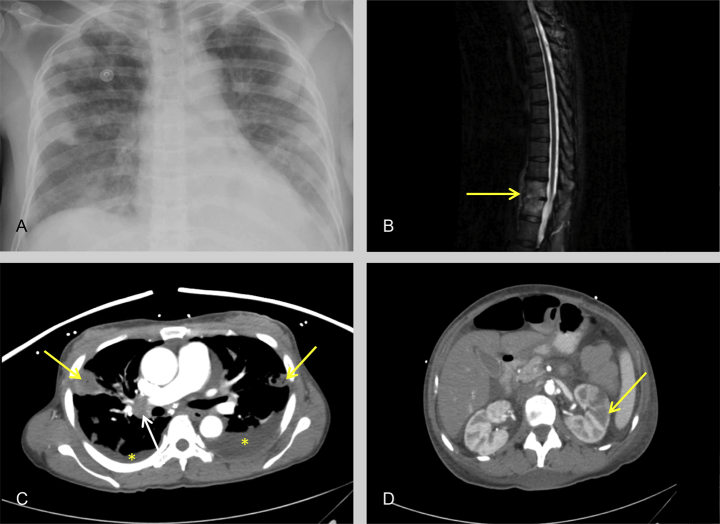
A 41-year-old woman with history of intravenous drug use presented with a two-week history of fever and fatigue. Physical examination revealed an erythematous, raised, and painful lesion on the lateral aspect of the left fifth finger, suggestive of an Osler's node (Figure 1A), and multiple small erythematous macular lesions on the sole of the right foot, suspicious for Janeway lesions (Figure 1B). Purpura, splinter hemorrhages, or conjunctival hemorrhages were not present. Cardiopulmonary auscultation was negative for murmurs, but diffuse crackles were audible in bilateral lung fields. The spine was tender to palpation in the lower thoracic and upper lumbar regions. Initial laboratory workup revealed leukocytosis and gram-positive cocci. Electrocardiogram did not reveal any arrhythmias or conduction abnormalities. Chest radiograph showed multifocal pneumonia with a small left-sided pleural effusion (Figure 2A). Empiric therapy with vancomycin, cefepime, and metronidazole was initiated.
Figure 1.
(A) An erythematous papular lesion on the lateral aspect of the left fifth digit represents an Osler's node. This is generally a round (1-2 cm in diameter), purplish, painful lesion that occurs most commonly on the fingertips or the lateral edges of the fingers, palms, toes, or soles and, histologically, is an area of sterile necrotizing vasculitis of the dermal glomus that is thought to be associated with immune complex deposition. (B) Small erythematous macular lesions on the sole of the right foot represent Janeway lesions. These are painless erythematous/hemorrhagic macular lesions on the palms and soles due to septic embolization to an area causing microabscess formation and thrombosis of small vessels in the area.
Figure 2.
(A) Anteroposterior chest radiograph showing multiple bilateral air space opacities and blunting of the left costophrenic angle. (B) Sagittal magnetic resonance T2 fat saturated image showing hyperintensity in the T11 and T12 vertebral bodies (yellow arrow) extending along the anterior aspect with loss of intervertebral disc space. These features are pathognomonic for diskitis or osteomyelitis. (C) Axial CT image of the chest (soft tissue window) with intravenous contrast in the arterial phase shows bilateral pleural effusions (asterisk), peripheral rounded opacities (yellow arrows) with central necrosis, characteristic for septic emboli, and enlarged right hilar node (white arrow). (D) Axial CT image of the abdomen with intravenous contrast in the arterial phase shows a wedge-shaped hypodensity (yellow arrow) in the left kidney causing blurring of the corticomedullary junction indicative of an infarct.
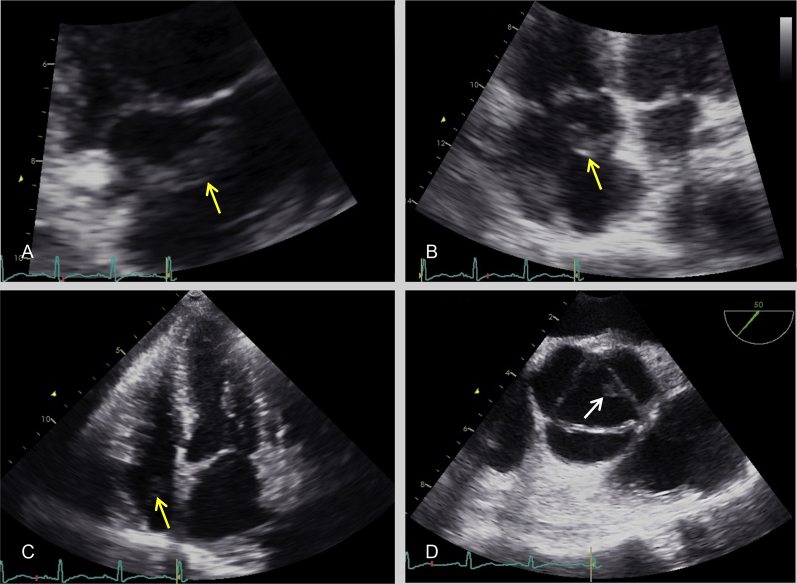
Transthoracic echocardiography (TTE) demonstrated a mobile echodensity (2.8 × 0.8 cm) in the right atrium (Figure 3 and Video 1) and moderate tricuspid regurgitation. Transesophageal echocardiography (TEE) confirmed the presence of a very large, highly mobile echodensity in the right atrium attached to the Chiari network and revealed an additional nodular echodensity (0.6 × 0.4 cm) on the left coronary cusp of the aortic valve (Figure 3D and Video 2) with mild aortic regurgitation. An atrial septal defect or patent foramen ovale was not identified.
Figure 3.
Right atrial echodensity (yellow arrows), measuring 2.8 × 0.8 cm in size, attached to the Chiari network demonstrated in the right ventricular inflow (A) and four-chamber (B and C) views by TTE. Aortic valve echodensity (white arrow), measuring 0.6 × 0.4 cm in size, attached to the margin of the left coronary cusp demonstrated on short-axis view (D) by TEE.
Magnetic resonance imaging (MRI) of the thoracic and lumbar spine showed enhancement of the T10, T11, and T12 vertebral bodies suggestive of osteomyelitis and diskitis without evidence of an epidural abscess (Figure 2B). MRI of the brain was negative for focal infectious lesions. Computed tomography (CT) of the chest revealed multiple cavitary lesions with thick walls and parenchymal consolidation in both lungs, suggestive of septic embolization (Figure 2C). CT of abdomen and pelvis showed multiple perfusion defects within both kidneys and a large area of hypoperfusion in the left kidney indicating renal infarcts (Figure 2D).
Blood cultures subsequently grew methicillin-resistant Staphylococcus aureus (MRSA). The patient was treated with intravenous vancomycin for 12 weeks and closely followed in the infectious disease, neurosurgery, and cardiology clinics after discharge from the hospital. Treatment course was complicated by Clostridium difficile diarrhea that was treated with metronidazole. The Osler's node and Janeway lesions resolved 8-10 weeks into therapy. At approximately 12 weeks of follow-up, TTE showed no evidence of vegetations on the Chiari network or the aortic valve, CT imaging indicated resolution of the lung and kidney lesions, and MRI of the spine to follow thoracic diskitis and osteomyelitis revealed evidence of resolving infection. There was no clinical evidence of recurrence at one year of follow-up.
Discussion
The Chiari network7 is a filamentous structure in the right atrium that is found in about 2% of patients undergoing TEE.8 This clinically benign structure can be confused with a pedunculated right atrial mass, thrombus, or a flail tricuspid leaflet.9 However, as noted in the present case, it may occasionally serve as a nidus of infection.5, 6
Right-sided infective endocarditis may spread to the left heart structures through a hematogenous route, which is likely in our patient. This dissemination may be facilitated by the presence of a right-to-left shunt at the intracardiac level (e.g., through patent foramen ovale)10 or intrapulmonary level (e.g., across pulmonary arteriovenous malformation or fistulae).11
The telltale dermatological features of infective endocarditis include Janeway lesions, Osler's nodes, purpura, splinter hemorrhages, and conjunctival hemorrhages.12 The cutaneous manifestations are due to embolic or immunologic phenomena and are associated with systemic embolization and predict a higher rate of complications.12 The incidence of cutaneous manifestations has been reduced due to early diagnosis and prompt treatment of bacteremia.12 However, these findings, if present at initial presentation, as in our patient, can provide important clinical clues to diagnose infective endocarditis.
Treatment of infective endocarditis involves the use of intravenous antibiotics and valve surgery, if indicated.13, 14 Most cases describing Chiari network endocarditis have reported use of surgery, as this filamentous network predisposes to the risk of persistent infection and thrombus formation.5, 6 In our case, MRSA bacteremia, vegetations on the Chiari network and aortic valve, and manifestations of pulmonary and systemic embolization resolved with prolonged antibiotic therapy.
Conclusions
In an injection drug user presenting with fever the prompt recognition of cutaneous signs (Janeway lesions and Osler's nodes) by simple visual inspection and expeditious demonstration of vegetations (on Chiari network and aortic valves) by echocardiography were fundamental in the rapid diagnosis of bilateral endocarditis. The ensuing diagnostic approach established the presence of methicillin-resistant staphylococcal endocarditis with septic emboli in both pulmonary and systemic end organs. Despite a very large vegetation (>2 cm in length) on the Chiari network that usually warrants early surgical removal, appropriate antimicrobial therapy alone resulted in complete resolution of all infectious manifestations with no residual sequelae.
Footnotes
Dr. Kenchaiah was partly supported by the Translational Research Institute, grant nos. UL1TR000039 and KL2TR000063 through the National Institutes of Health (NIH) National Center for Research Resources and National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.01.007.
Supplementary Data
TTE demonstrating a very large, highly mobile echodensity, measuring 2.8 × 0.8 cm in size, attached to the Chiari network in the right atrium. The mass was noted to prolapse across the tricuspid valve annulus into the right ventricle during diastole.
TEE showing a small rounded echodensity, measuring 0.6 × 0.4 cm in size, attached to the left coronary cusp of the aortic valve.
References
- 1.Weisse A.B., Heller D.R., Schimenti R.J., Montgomery R.L., Kapila R. The febrile parenteral drug user: a prospective study in 121 patients. Am J Med. 1993;94:274–280. doi: 10.1016/0002-9343(93)90059-x. [DOI] [PubMed] [Google Scholar]
- 2.Mathew J., Addai T., Anand A., Morrobel A., Maheshwari P., Freels S. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug users. Arch Intern Med. 1995;155:1641–1648. [PubMed] [Google Scholar]
- 3.Cherubin C.E., Baden M., Kavaler F., Lerner S., Cline W. Infective endocarditis in narcotic addicts. Ann Intern Med. 1968;69:1091–1098. doi: 10.7326/0003-4819-69-6-1091. [DOI] [PubMed] [Google Scholar]
- 4.Levine D.P., Crane L.R., Zervos M.J. Bacteremia in narcotic addicts at the Detroit Medical Center. II. Infectious endocarditis: a prospective comparative study. Rev Infect Dis. 1986;8:374–396. doi: 10.1093/clinids/8.3.374. [DOI] [PubMed] [Google Scholar]
- 5.Payne D.M., Baskett R.J., Hirsch G.M. Infectious endocarditis of a Chiari network. Ann Thorac Surg. 2003;76:1303–1305. doi: 10.1016/s0003-4975(03)00522-8. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi N., Bhagirath K., Ariyarajah V., Fang T., Ahmadie R., Lytwyn M. Chiari network endocarditis: not just an innocent bystander. Echocardiography. 2008;25:642–645. doi: 10.1111/j.1540-8175.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiari H. Ueber Netzbildungen im rechten Vorhofe des Herzens. Beitr Pathol Anat. 1897;22:1–10. [Google Scholar]
- 8.Schneider B., Hofmann T., Justen M.H., Meinertz T. Chiari's network: normal anatomic variant or risk factor for arterial embolic events? J Am Coll Cardiol. 1995;26:203–210. doi: 10.1016/0735-1097(95)00144-o. [DOI] [PubMed] [Google Scholar]
- 9.Werner J.A., Cheitlin M.D., Gross B.W., Speck S.M., Ivey T.D. Echocardiographic appearance of the Chiari network: differentiation from right-heart pathology. Circulation. 1981;63:1104–1109. doi: 10.1161/01.cir.63.5.1104. [DOI] [PubMed] [Google Scholar]
- 10.Johri A.M., Kovacs K.A., Kafka H. An unusual case of infective endocarditis: extension of a tricuspid valve vegetation into the left atrium through a patent foramen ovale. Can J Cardiol. 2009;25:429–431. doi: 10.1016/s0828-282x(09)70515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negi S.I., Anand A. Widespread systemic embolization with isolated tricuspid valve endocarditis. Heart Lung. 2012;41:387–389. doi: 10.1016/j.hrtlng.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Servy A., Valeyrie-Allanore L., Alla F., Lechiche C., Nazeyrollas P., Chidiac C. Prognostic value of skin manifestations of infective endocarditis. JAMA Dermatol. 2014;150:494–500. doi: 10.1001/jamadermatol.2013.8727. [DOI] [PubMed] [Google Scholar]
- 13.Baddour L.M., Wilson W.R., Bayer A.S., Fowler V.G., Jr., Tleyjeh I.M., Rybak M.J. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 14.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE demonstrating a very large, highly mobile echodensity, measuring 2.8 × 0.8 cm in size, attached to the Chiari network in the right atrium. The mass was noted to prolapse across the tricuspid valve annulus into the right ventricle during diastole.
TEE showing a small rounded echodensity, measuring 0.6 × 0.4 cm in size, attached to the left coronary cusp of the aortic valve.