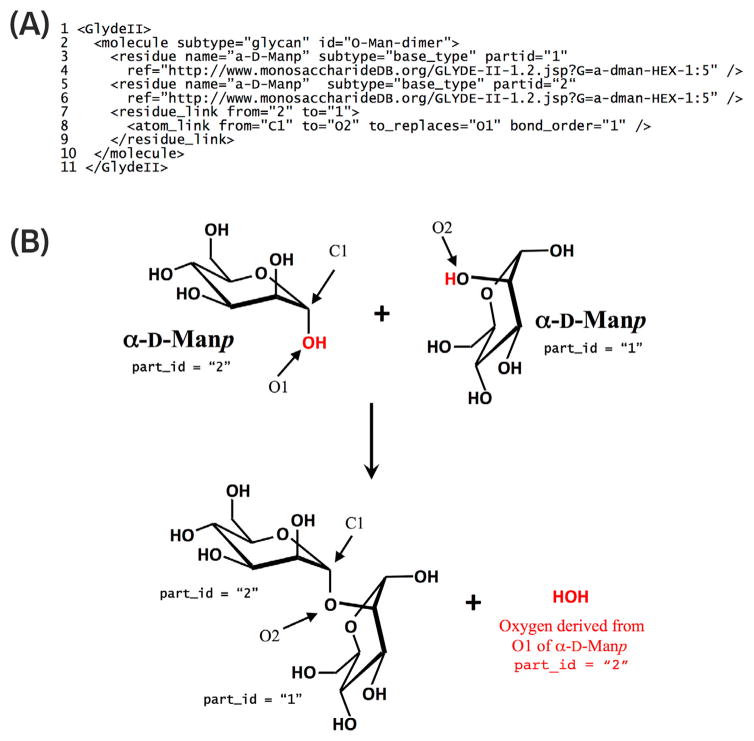
Figure 4.
(A) GLYDE-II representation of the disaccharide molecule that is used as an archetype for the disaccharide moiety shown in Fig. 5. The properties of each of the residue objects in this molecule are specified by reference to the archetypal α-D-Manp molecule shown in Fig. 3. The residue link (line 7) formally indicates a directed bond from one residue to another. This residue link, in turn, encompasses an atom link (line 8) from C1 of one of the α-D-Manp residue objects (part id = “2”) to O2 of the other α-D-Manp residue object (part id = “1”). These directional semantics allow unambiguous interpretation of the attributes (e.g., from = “C1” and to = “O2”) in the atom link. That is, the bond extends from “C1” of α-D-Manp residue (part id = “1”) to “O2” of α-D-Manp residue (part id = “2”). This code also infers that, in the context of this atom link, “to” is a synonym for “O2 of α-D-Manp residue #1” and “from” is a synonym for “C1 of α-D-Manp residue #2”. By rule, an atom in one residue can replace an atom in the other residue when a residue link is formed (see Panel B). The atom link attribute to replaces = “O1” can thus be unambiguously interpreted as “O2 of α-D-Manp residue #1 replaces O1 of α-D-Manp residue #2”. (B) Formation of the glycosidic bond connecting the two α-D-Manp residue objects in the disaccharide encoded by the text in (A). The GLYDE convention dictates that the geometry of each archetypal monosaccharide molecule is retained when it is transformed into a residue. During this transformation, the glycosidic oxygen of one residue (e.g. O1 of the α-D-Manp residue #2) is released as a water molecule and replaced by an atom in the other residue (e.g. O2 of α-D-Manp residue #1).