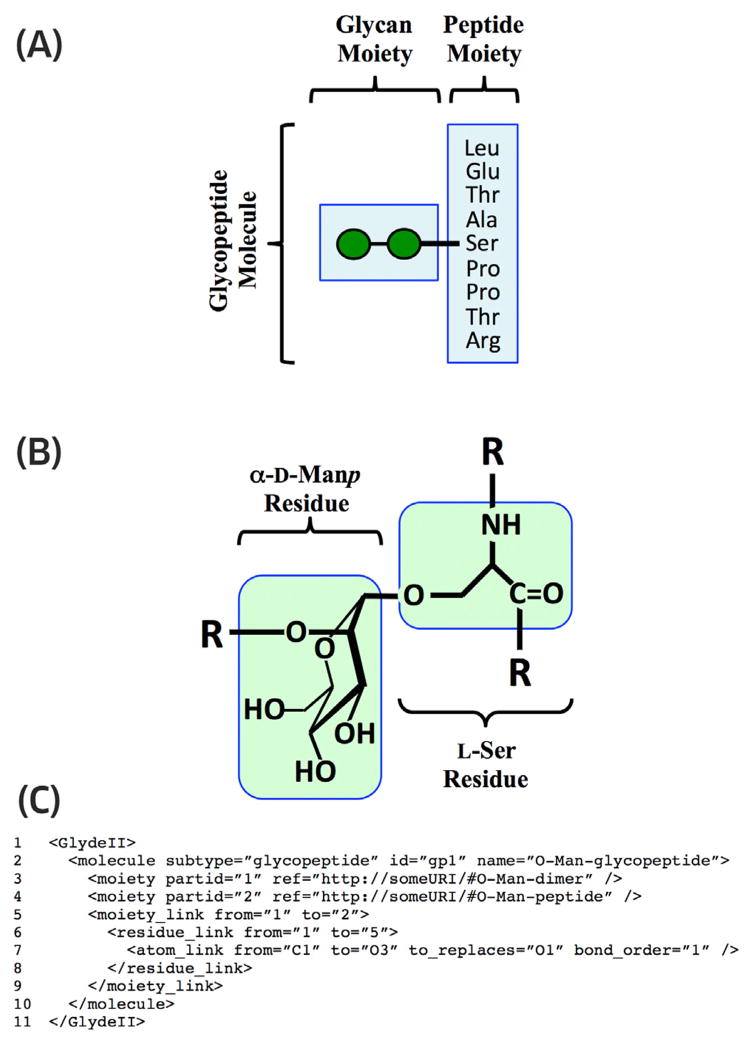
Figure 5.
The structure of a glycopeptide molecule illustrated as a hierarchical collection of GLYDE parts (bound atom, residue and moiety) connected by links. Residues in the glycan moiety are represented using the CFG graphical format for glycan structure. (A) The glycan moiety and peptide moiety are connected by a moiety link, which embodies a residue link connecting one of the α-D-Manp residue objects (green circle) in the gly-can moiety to the Ser residue in the peptide moiety. (B) The two residues that connect the moieties are shown in atomic detail. The residue link connecting these residues embodies an atom link connecting C1 of the α-D-Manp residue to O3 of the Ser residue. (C) GLYDE-II representation of the glycopeptide. The moiety link indicates that the “O-Man-dimer” (Fig. 4) is covalently attached to the “O-Man-peptide”, whose structure is specified in “http://someURI”. The enclosed residue link indicates that the α-D-Manp residue (partid = “1” in the GLYDE-II specification of the “O-Man-dimer”) is linked to the L-Ser residue (partid = “5” in the GLYDE-II specification of the “O-Man-peptide”). The further enclosed atom link indicates that the bound atom C1 (partid = “C1” in the referenced GLYDE-II specification of α-D-Manp residue objects in the “O-Man-dimer” moiety) is covalently attached to the bound atom O3 (par-tid = “O3” in the referenced GLYDE-II specification of the L-Ser residue objects in the “O-Man-peptide”).