Abstract
Lateral roots (LRs), which originate from the growing root, and adventitious roots (ARs), which are formed from non-root organs, are the main contributors to the post-embryonic root system in Arabidopsis. However, our knowledge of how formation of the root system is altered in response to diverse inductive cues is limited. Here, we show that WOX11 contributes to root system plasticity. When seedlings are grown vertically on medium, WOX11 is not expressed in LR founder cells. During AR initiation, WOX11 is expressed in AR founder cells and activates LBD16. LBD16 also functions in LR formation and is activated in that context by ARF7/19 and not by WOX11. This indicates that divergent initial processes that lead to ARs and LRs may converge on a similar mechanism for primordium development. Furthermore, we demonstrated that when plants are grown in soil or upon wounding on medium, the primary root is able to produce both WOX11-mediated and non-WOX11-mediated roots. The discovery of WOX11-mediated root-derived roots reveals a previously uncharacterized pathway that confers plasticity during the generation of root system architecture in response to different inductive cues.
KEY WORDS: Adventitious root, Lateral root, LBD16, WOX11, Arabidopsis, Root system
Summary: Root system development can respond flexibly to developmental and environmental cues by utilizing WOX11-mediated and non-WOX11-mediated pathways, which converge on a common mechanism for primordium development involving LBD16.
INTRODUCTION
The plant root system is a complex result of iterative developmental processes, including formation of the primary root during embryogenesis and production of new root primordia after germination, including lateral roots (LRs) and adventitious roots (ARs) (Lavenus et al., 2013; Atkinson et al., 2014; Bellini et al., 2014). In Arabidopsis thaliana, LRs are initiated from the xylem-pole pericycle cells of a growing root in a developmentally and periodically organized pattern (Peret et al., 2009; Moreno-Risueno et al., 2010; Lavenus et al., 2013; Van Norman et al., 2014; Xuan et al., 2015, 2016), whereas ARs are described as being formed in non-root organs such as the hypocotyl and detached stem and leaf explants primarily in response to environmental cues, such as wounding or stress (Bellini et al., 2014; Xu and Huang, 2014; Rellan-Alvarez et al., 2016). However, how the root system is plastically formed in response to diverse inductive cues when plants are grown in different conditions is barely understood.
The cellular and molecular processes key to LR formation from the primary root grown vertically on medium have been extensively investigated (de Smet, 2012; Laskowski, 2013; Vermeer and Geldner, 2015). Generally, an oscillatorily root cap-derived flux of auxin signaling is thought to prime the LR founder cells (Moreno-Risueno et al., 2010; Xuan et al., 2015, 2016) which activates AUXIN RESPONSE FACTOR7 (ARF7) and ARF19 (Okushima et al., 2007) transcription factors. In turn, ARF7 and ARF19 upregulate LATERAL ORGAN BOUNDARIES DOMAIN (LBD) genes for the initiation of a lateral root primordium (LRP) (Okushima et al., 2007; Lee et al., 2009; Goh et al., 2012).
We previously reported that WUSCHEL-RELATED HOMEOBOX11 (WOX11) is required for de novo regeneration of ARs from leaf explants (Liu et al., 2014), raising the possibility that WOX11 is involved in AR initiation. In the current study, we show that WOX11 is not involved in LR initiation when plants are vertically grown on medium, and that the primary root can produce both WOX11-mediated roots and ARF7/19-mediated LRs in response to different inductive cues when plants are grown in soil or upon wounding on medium. The different root system architecture of plants grown in different conditions suggests flexibility of rooting pathways in response to developmental and environmental cues.
RESULTS
WOX11 does not contribute to LR initiation when plants are vertically grown on medium
To analyze the role of WOX11, we exploited adventitious rooting systems in Arabidopsis from leaf explants (Fig. 1A-D; Fig. S1A,E,I), stem explants (Fig. 1A; Fig. S1B,F,J) and hypocotyls (Fig. 1A; Fig. S1C,G,K). Detached leaf and stem explants generate ARs within 8 and 6 days after culture (DAC) on B5 medium, respectively (Chen et al., 2014). We also observed lateral rooting from the primary root grown vertically on medium (Fig. 1A,E-G; Fig. S1D,H,L). All plants and explants were grown and cultured in vitro on medium.
Fig. 1.
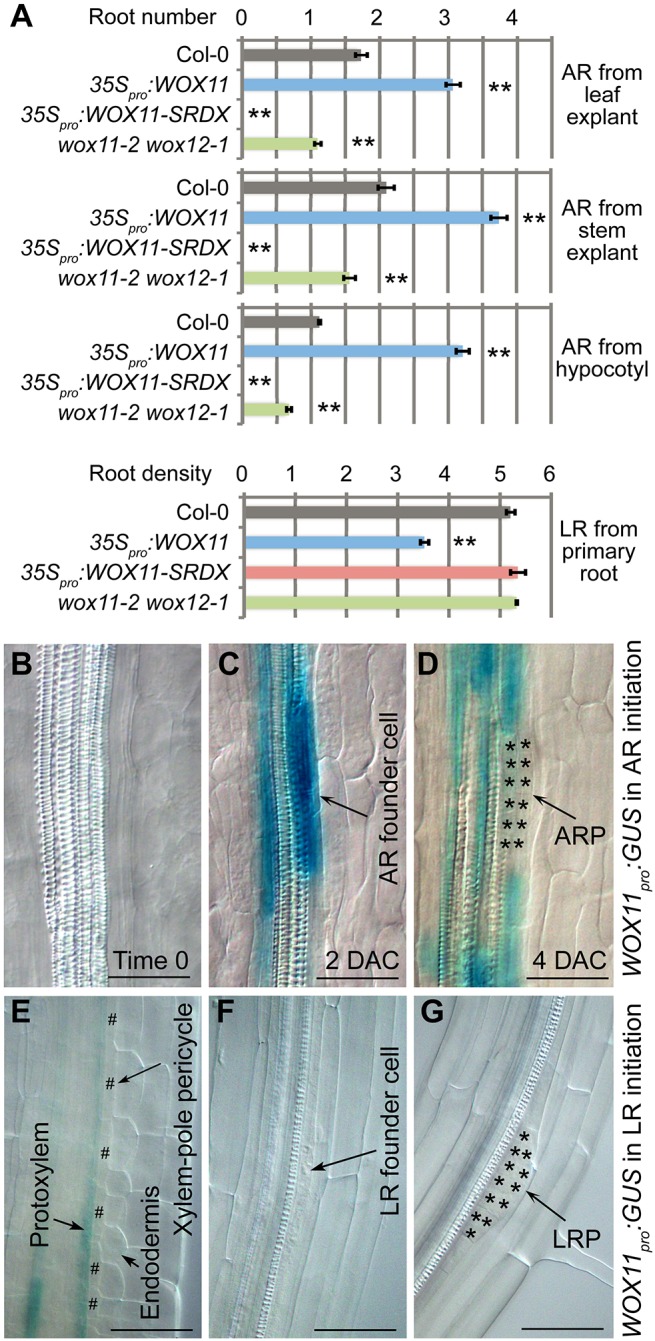
Analysis of WOX11 in AR and LR formation on medium. (A) Statistical analysis of root number per leaf explant at 12 DAC, per stem explant at 12 DAC, per hypocotyl and per 1-cm primary root. LR analysis includes emerged LRs together with LRP observed under DIC microscopy. Error bars represent s.e.m. from three biological repeats. n=30 (leaf explant, stem explant and hypocotyl) or n=10 (primary root) in each repeat. **P<0.01 (two-sample Student's t-test compared with Col-0). (B-D) GUS staining of WOX11pro:GUS from leaf explant at time 0 (B), 2 DAC (C) and 4 DAC (D). Note that the GUS signal was detected specifically in the AR founder cells (C) (Liu et al., 2014). We observed more than 20 leaf explants from two independent lines and all of them showed GUS staining in AR founder cells. (E-G) GUS staining of WOX11pro:GUS in the primary root. Note that GUS signal was detected in the protoxylem at the meristematic region (E) as previously reported (Liu et al., 2014), but was not detected in xylem-pole pericycle cells (E), LR founder cells (F) or LRP (G). We observed more than 20 seedlings from two independent lines and none of them showed GUS staining in LR founder cells. Also see Fig. S4D-F. Leaf explants (A-D) and stem explants (A) were detached from 12- and 24-day-old seedlings grown on 1/2 MS medium, respectively. The detached explants were then cultured on B5 medium without added hormones (Chen et al., 2014). Hypocotyls (A) were analyzed using 14-day-old seedlings vertically grown on 1/2 MS medium. Primary and lateral roots were analyzed using 9-day-old (A) or 7-day-old (E-G) seedlings vertically grown on 1/2 MS medium. Asterisks in D and G indicate root primordium cells, and hash symbols in E indicate xylem-pole pericycle. Scale bars: 50 μm.
We examined the role of WOX11 in these rooting systems using two transgenic constructs, 35Spro:WOX11 and 35Spro:WOX11-SRDX, to enhance and block WOX11 activity, respectively (Liu et al., 2014). Compared with wild-type Columbia-0 (Col-0) (Fig. 1A; Fig. S1A-C), AR formation was greatly enhanced in leaf explants, stem explants and hypocotyls of 35Spro:WOX11 plants (Fig. 1A; Fig. S1E-G), whereas AR production was severely inhibited in 35Spro:WOX11-SRDX plants (Fig. 1A; Fig. S1I-K). However, compared with Col-0, no defects in LR density in 35Spro:WOX11-SRDX or enhanced LR density in 35Spro:WOX11 were observed (Liu et al., 2014) (Fig. 1A; Fig. S1D,H,L), although 35Spro:WOX11-SRDX had a shorter primary root than Col-0 (Fig. S2). Instead, LR density in 35Spro:WOX11 plants was reduced (Fig. 1A; Fig. S1H). This response might be caused by a feedback process due to enhanced adventitious rooting of hypocotyls induced by WOX11 overexpression (Fig. 1A; Fig. S1G).
WOX11 and WOX12 are partially redundant genes (Liu et al., 2014). To understand further the roles of WOX11 and WOX12 in adventitious rooting, we analyzed the phenotype of a wox11-2 wox12-1 double mutant. Adventitious rooting from leaf explants, stem explants and hypocotyls were defective in wox11-2 wox12-1 compared with Col-0 (Fig. 1A), although the defects were milder than those in 35Spro:WOX11-SRDX plants. In contrast to the defective adventitious rooting, lateral root density remained normal in the wox11-2 wox12-1 mutant (Fig. 1A).
Next, we compared the expression pattern of WOX11 during AR and LR formation using WOX11pro:GUS transgenic lines (Liu et al., 2014). WOX11 promoter activity was confirmed by introducing WOX11pro:WOX11 into the wox11-2 wox12-1 background, which complemented the adventitious rooting defect (Fig. S3). WOX11 was induced in all examined AR-competent tissues during AR formation (Fig. S4A-C). A detailed analysis using leaf explants showed that WOX11 was initially induced in AR founder cells within 2 DAC (Fig. 1B,C; Fig. S4J,K) (Liu et al., 2014). Expression of WOX11 then gradually decreased in the adventitious root primordium (ARP) as it developed (Fig. 1D; Fig. S4L) (Liu et al., 2014).
In contrast, GUS signal was not detectable during LR formation (Fig. S4D-F). More specifically, WOX11 expression was absent in xylem-pole pericycle cells (Fig. 1E; Fig. S4E,F), LR founder cells (Fig. 1F; Fig. S4E,F) and LRP (Fig. 1G; Fig. S4E,F). Instead, expression was detected in protoxylem cells in the primary root meristematic region (Fig. 1E; Fig. S4E) (Liu et al., 2014). In addition, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis confirmed the expression of WOX11 during AR but not LR formation (Fig. S4G-I). Therefore, LR formation from the primary root grown vertically on medium is controlled by the non-WOX11-mediated pathway.
In addition, we performed experiments in different growth conditions and confirmed that WOX11 was also involved in light control of adventitious rooting from hypocotyls (Sorin et al., 2005; Gutierrez et al., 2009) (Fig. S5). We tested WOX11 expression patterns in leaf explants and primary roots by culturing them on different medium, and the results showed that change of the medium had little effect on WOX11 expression (Fig. S6).
In summary, WOX11 was specifically expressed in diverse adventitious rooting systems but not in LR formation from the primary root grown vertically on medium. Therefore, when plants are vertically grown on medium, lateral rooting follows the non-WOX11-mediated pathway, which requires ARF7/19.
The primary root is able to produce both WOX11-mediated roots and non-WOX11-mediated roots when plants are grown in soil or upon wounding
According to traditional views, Arabidopsis LRs derive from xylem-pole pericycle cells of an existing root and the initiation of all LRs is usually considered to be dependent on identical processes. Here, we re-examined ‘lateral root’ formation by analyzing whether WOX11-mediated roots can develop from the primary root.
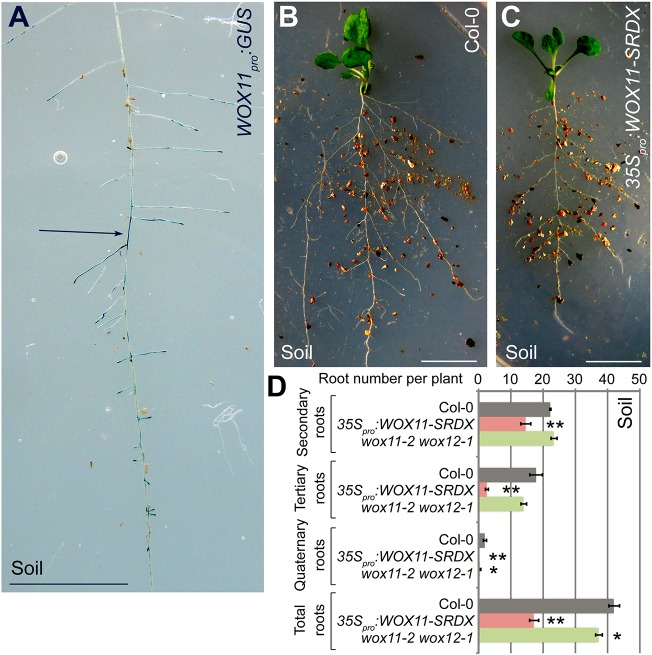
LR initiation from wild-type seedlings grown vertically on half-strength Murashige and Skoog (1/2 MS) medium or some other media usually did not display a WOX11 signal and followed the non-WOX11-mediated rooting pathway (Fig. 1E-G; Fig. S4D-F; Fig. S6). However, in natural conditions, plants are grown in soil. Therefore, we tested whether the root system grown in soil conditions is able to produce WOX11-mediated roots. Strikingly, in WOX11pro:GUS plants grown in soil, GUS signal was detected in many regions of the root system (Fig. 2A; Fig. S7). In addition, secondary, tertiary and quaternary root numbers were all reduced in the root system of 35Spro:WOX11-SRDX seedlings grown in soil compared with those of Col-0 (Fig. 2B-D). Although 35Spro:WOX11-SRDX had a shorter primary root than Col-0 (Fig. S2), the density of the secondary roots derived from the primary roots was also reduced in 35Spro:WOX11-SRDX seedlings grown in soil compared with those of Col-0 (Fig. S8). In wox11-2 wox12-1 seedlings grown in soil, the number of quaternary roots was reduced compared with those of Col-0 (Fig. 2D). In 35Spro:WOX11 seedlings grown in soil, the numbers of tertiary and quaternary roots were enhanced compared with those of Col-0 (Fig. S9). Taken together, these data suggest that in soil conditions, WOX11-mediated root formation does occur in the root system, which may reflect a response to environmental signals in soil.
Fig. 2.
WOX11-mediated rooting contributes to the root system derived from the primary root in soil. (A) GUS staining of an 18-day-old WOX11pro:GUS plant grown in soil. The GUS signal was observed in many regions of the root (arrow). Seedlings were carefully moved out of the pot, together with the soil that was adhered to the root, for GUS staining. After GUS staining, the soil was washed away before observation. (B,C) The primary root and its root system from 18-day-old Col-0 (B) and 35Spro:WOX11-SRDX (C) grown in soil. (D) Statistical analysis of the root systems of 18-day-old Col-0, 35Spro:WOX11-SRDX and wox11-2 wox12-1 plants grown in soil. Error bars represent s.e.m. from three biological repeats. n=10 plants for each repeat. *P<0.05, **P<0.01 (two-sample Student's t-test compared with Col-0). Also see Fig. S8. Scale bars: 1 cm.
When plants are grown in soil, they may encounter different nutrition conditions, different types of stresses, damage of roots, and interactions with microbe and insects. We investigated whether damage of the primary root is able to induce WOX11-mediated root formation. The primary root of 12-day-old WOX11pro:GUS seedlings vertically grown on 1/2 MS medium was cut at the midpoint to mimic damage of roots. GUS signals could be detected at the excision site within 1 day after excision (DAE) (Fig. S10), suggesting that WOX11-mediated root initiation might occur upon excision of the wild-type primary root tip.
To eliminate interference from non-WOX11-mediated roots, i.e. ARF7/19-mediated LRs, we performed experiments using the arf7-1 arf19-1 double mutant, which is defective in LR initiation when plants are grown vertically on 1/2 MS medium (Fig. 3A) (Okushima et al., 2007). The primary root of 12-day-old arf7-1 arf19-1 seedlings vertically grown on 1/2 MS medium was cut at the midpoint to mimic damage of roots (Fig. 3A). Surprisingly, many clusters of roots were formed near the wounded region at 6 DAE (Fig. 3B). Using an auxin reporter DR5pro:GUS line, we observed that GUS staining accumulated in the wounded region at 1 DAE (Fig. 3C). In WOX11pro:GUS-marked arf7-1 arf19-1 roots, GUS signal was detected in pericycle cells and some other vascular cells in the wounded region at 1 DAE (Fig. 3D). Moreover, introgression of 35Spro:WOX11-SRDX into arf7-1 arf19-1 resulted in defective rooting from the wounded primary root at 6 DAE (Fig. 3E). These data indicate that the roots from the wounded arf7-1 arf19-1 primary root were formed following the WOX11-mediated rooting pathway rather than the ARF7/19-mediated LR pathway.
Fig. 3.
Root formation from the arf7-1 arf19-1 primary root upon wounding. (A) The arf7-1 arf19-1 mutant shows defective LR initiation. (B) Formation of roots (arrow) on wounded primary root of arf7-1 arf19-1 at 6 DAE. (C,D) GUS staining of DR5pro:GUS (C) and WOX11pro:GUS (D) in the wounded primary root of arf7-1 arf19-1 at 1 DAE. (E) Repression of root formation on wounded primary root of arf7-1 arf19-1 in the 35Spro:WOX11-SRDX background at 6 DAE. (F) Decapitation by removal of the above-ground tissues including the shoot and the hypocotyl resulted in loss of rooting capability on the wounded primary root of arf7-1 arf19-1 at 6 DAE. (G,H) GUS staining of DR5pro:GUS (G) and WOX11pro:GUS (H) in the wounded primary root of arf7-1 arf19-1 with decapitation (indicated in F) at 1 DAE. (I) Rescue of the rooting capability (arrows) by application of 1 μM indole-3-acetic acid in an agar mass to the decapitated region (indicated in F). Twelve-day-old seedlings vertically grown on 1/2 MS medium were used for analysis. Scissors indicate the cutting sites. Scale bars: 1 mm (A,B,E,F,I); 50 μm (C,D,G,H).
When the above-ground portion was removed from the junction between root and hypocotyl, the remaining arf7-1 arf19-1 primary root grown vertically on 1/2 MS medium lost the ability to form roots upon wounding (Fig. 3F), and GUS staining was barely observed near the wounded region from the cut in the primary roots from both DR5pro:GUS/arf7-1 arf19-1 and WOX11pro:GUS/arf7-1 arf19-1 seedlings (Fig. 3G,H). The application of auxin specifically to the decapitated region at the hypocotyl-root junction of arf7-1 arf19-1 could partially rescue the rooting defect, as the cut region of the primary root retained the ability to develop roots (Fig. 3I). These results suggest that long-distance transport of auxin from above-ground tissues (i.e. basipetal auxin flux) is required for WOX11-mediated root formation in the arf7-1 arf19-1 primary root upon wounding.
Furthermore, many roots can be formed from the primary root of arf7-1 arf19-1 in soil (Fig. 4A) and these roots were formed following the WOX11-mediated rooting pathway (Fig. 4B-D). Drought conditions may also promote root formation from the primary root of arf7-1 arf19-1 vertically grown on 1/2 MS medium (Fig. 5A) and induce WOX11 expression in the primary root of arf7-1 arf19-1 (Fig. 5B,C).
Fig. 4.
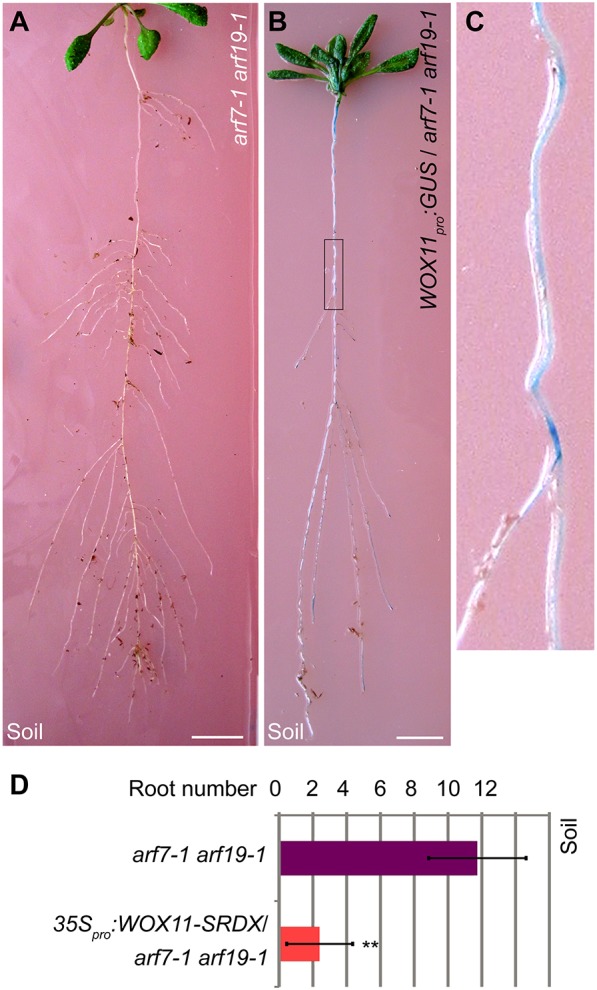
Root formation of arf7-1 arf19-1 in soil. (A) The primary root and its root system from 18-day-old arf7-1 arf19-1 grown in soil. (B) GUS staining of the primary root and its root system from 18-day-old WOX11pro:GUS/arf7-1 arf19-1 grown in soil. (C) Magnification of the boxed region in B showing GUS signal in more detail. (D) Statistical analysis of root number from the primary root of 18-day-old arf7-1 arf19-1 or 35Spro:WOX11-SRDX/arf7-1 arf19-1 grown in soil. Error bars represent s.e.m. from three biological repeats. n=10 for each repeat. **P<0.01 (two-sample Student's t-test compared with arf7-1 arf19-1). Scale bars: 1 cm (A,B).
Fig. 5.
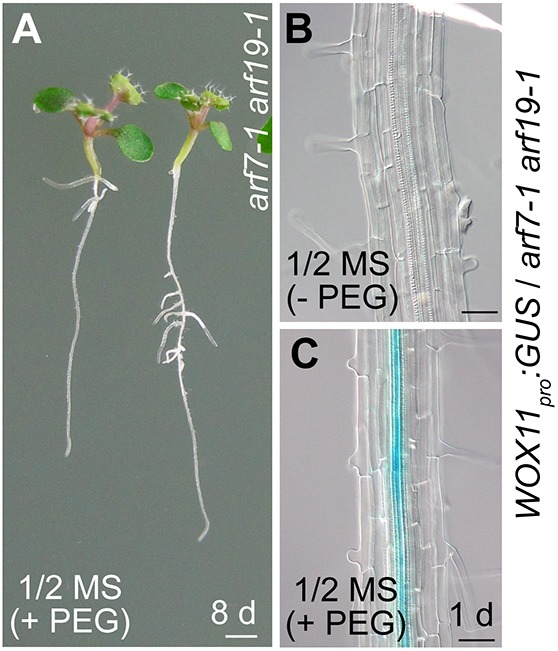
The primary root and its root system from arf7-1 arf19-1 under drought conditions. (A) Root formation from the primary root and the hypocotyl of arf7-1 arf19-1 under polyethylene glycol (PEG) treatment. (B,C) GUS staining of the primary root from WOX11pro:GUS/arf7-1 arf19-1 before PEG treatment (−PEG; B) and after PEG treatment (+PEG; C). The plants were grown vertically on 1/2 MS medium after germination for 6 days (B) and then transferred to 1/2 MS medium containing 5% PEG to mimic the drought condition (Verslues et al., 2006) for 8 days (A) or 1 day (C). Scale bars: 1 mm (A); 50 μm (B,C).
Taken together, our results reveal a novel root-derived root induction process, which extends the plasticity of root system architecture.
WOX11-mediated and non-WOX11-mediated root initiation converges on the activation of LBD16
Direct activation of LBD16 by ARF7 and ARF19 is a key step during LRP initiation in the non-WOX11-mediated rooting pathway (Okushima et al., 2007; Lee et al., 2009; Goh et al., 2012). We observed that a LBD16 loss-of-function mutant, lbd16-2 (Fan et al., 2012), was defective in AR formation (Fig. 6A-C) and, to a lesser extent, in LR formation (Fig. 6D) when grown vertically on medium, suggesting that LBD16 is involved in both WOX11-mediated and non-WOX11-mediated rooting pathways.
Fig. 6.
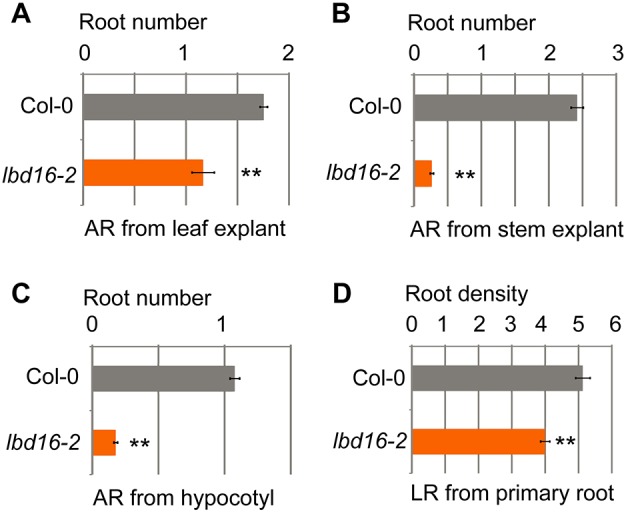
LBD16 involvement in lateral and adventitious rooting. (A-D) Statistical analyses of root number per leaf explant (A), per stem explant (B), per hypocotyl (C) and per 1-cm primary root (D). Error bars represent s.e.m. with three biological repeats. n=30 (leaf explant, stem explant and hypocotyl) or n=10 (primary root) in each repeat. **P<0.01 (two-sample Student's t-test compared with Col-0). The plant material conditions were the same as described in Fig. 1A.
Genetic data showed that WOX11 acted upstream of LBD16 during AR formation from leaf explants (Liu et al., 2014) (Fig. S11). Here, we used several approaches to test whether WOX11 is able to regulate LBD16 directly during AR formation from leaf explants.
We first analyzed the promoter sequence of LBD16 and noted that it contains two WOX-binding cis elements (TTAATGG) (Lohmann et al., 2001; Leibfried et al., 2005; Zhao et al., 2009) (Fig. 7A). Results from chromatin immunoprecipitation (ChIP) analysis showed that the 3×FLAG-WOX11-GR protein, in which the WOX11 protein was fused with the 3×FLAG tag and a dexamethasone (DEX)-induced nuclear localization domain GLUCOCORTICOID RECEPTOR (GR) (Aoyama and Chua, 1997), was able to directly bind the promoter of LBD16 at the predicted binding sites in 35Spro:3×FLAG-WOX11-GR transgenic plants (Fig. 7B).
Fig. 7.
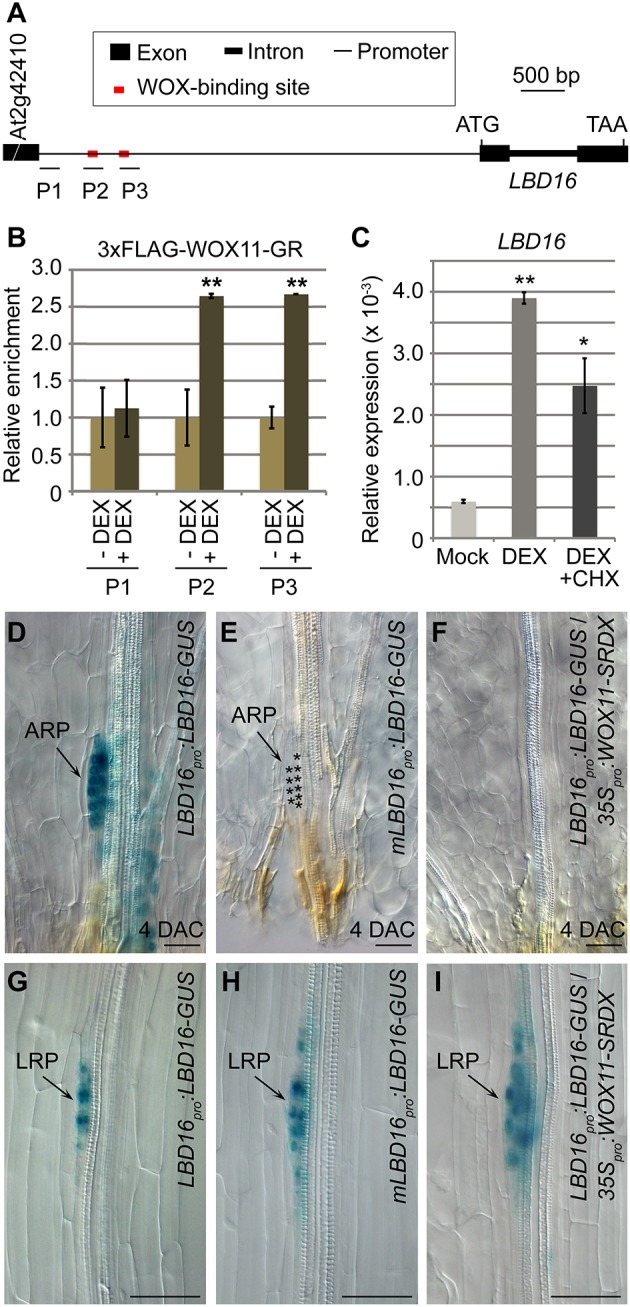
WOX11 directly upregulates LBD16 in AR initiation. (A) Schematic of the structure of the LBD16 gene. Horizontal lines below the gene show positions of PCR fragments in the ChIP analysis shown in B. (B) ChIP analysis showed that 3×FLAG-WOX11-GR was enriched in the promoter of LBD16. Twelve-day-old seedlings grown on 1/2 MS medium were treated with 10 μM DEX (+DEX) or DMSO mock (–DEX) for 4 h. Petioles were harvested for ChIP analysis because petioles contain an abundance of cells competent for adventitious rooting. The ChIP results were normalized to the input control. The values in –DEX were arbitrarily fixed at 1.0. **P<0.01 (two-sample Student's t-test compared with –DEX). (C) qRT-PCR analysis of LBD16 expression in 35Spro:3×FLAG-WOX11-GR. Leaf explants detached from 12-day-old seedlings were treated with DMSO (mock), 10 μM DEX, or both 10 μM DEX and 10 μM CHX on B5 medium for 4 h for RNA extraction. *P<0.05, **P<0.01 (two-sample Student's t-test compared with the mock). (D-F) GUS staining of LBD16pro:LBD16-GUS (D), mLBD16pro:LBD16-GUS (E) and LBD16pro:LBD16-GUS/35Spro:WOX11-SRDX (F) leaf explants cultured on B5 medium at 4 DAC. Leaf explants were detached from 12-day-old seedlings grown on 1/2 MS medium. Asterisks in E indicate ARP cells. (G-I) GUS staining of primary roots from 9-day-old LBD16pro:LBD16-GUS (G), mLBD16pro:LBD16-GUS (H) and LBD16pro:LBD16-GUS/35Spro:WOX11-SRDX (I) grown vertically on 1/2 MS medium. Error bars represent s.e.m. from three biological repetitions. Each biological repetition was performed with three technical repetitions. Two independent transgenic lines of either LBD16pro:LBD16-GUS or mLBD16pro:LBD16-GUS were tested in D-I and the results were consistent. Scale bars: 50 μm.
Upon DEX treatment, LBD16 expression was upregulated in leaf explants carrying the 3×FLAG-WOX11-GR construct (Fig. 7C). Moreover, this upregulation of LBD16 remained when treated with DEX together with cycloheximide (CHX), an inhibitor of protein synthesis, indicating the direct regulation of LBD16 by WOX11 (Fig. 7C).
To determine the functionality of the identified WOX-binding sites, we compared GUS staining in two reporter lines: one harboring an LBD16pro:LBD16-GUS fusion and the other carrying the LBD16 promoter mutated at two WOX-binding sites (mLBD16pro:LBD16-GUS). GUS staining of LBD16pro:LBD16-GUS was induced during ARP initiation in leaf explants at 4 DAC (Fig. 7D), whereas GUS staining was barely detected in the mLBD16pro:LBD16-GUS leaf explants at 4 DAC (Fig. 7E). Moreover, in LBD16pro:LBD16-GUS/35Spro:WOX11-SRDX leaf explants, GUS staining was not detected (Fig. 7F). These data suggest that WOX11 acts as a transcriptional activator of LBD16 mainly through direct binding to the WOX-binding sites during ARP initiation.
On the other hand, during LRP initiation from the primary root grown vertically on medium, the GUS signals of LBD16pro:LBD16-GUS (Fig. 7G), mLBD16pro:LBD16-GUS (Fig. 7H) and LBD16pro:LBD16-GUS/35Spro:WOX11-SRDX (Fig. 7I) were all clearly elevated. This suggests that WOX11 does not regulate LBD16 in lateral rooting when plants are grown vertically on 1/2 MS medium.
In summary, our data strongly support the hypothesis that WOX11 directly activates LBD16 expression in the WOX11-mediated rooting pathway. ARF7/19 activate LBD16 expression in LR initiation in the non-WOX11-mediated rooting pathway when plants are grown vertically on medium. Therefore, WOX11-mediated and non-WOX11-mediated rooting pathways may eventually converge to the activation of LBD16.
DISCUSSION
Based on the present results, we provide a model for the WOX11-mediated and non-WOX11-mediated rooting pathways in Arabidopsis root system formation (Fig. 8).
Fig. 8.
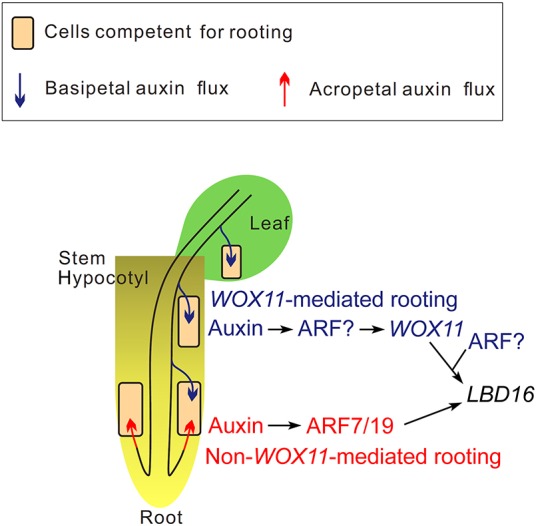
Model of root system formation in Arabidopsis. WOX11-mediated (blue) and non-WOX11-mediated (red) rooting pathways contribute to the plasticity of Arabidopsis root system formation. WOX11-mediated rooting can occur in leaf explants, stem explants and hypocotyls. WOX11-mediated rooting can also occur in the root mainly in response to environmental signals, such as wounding or stress. The non-WOX11-mediated rooting pathway is dependent on developmental signals to produce LRs. The question marks indicate the proposal that ARF proteins are involved in WOX11-mediated rooting.
When plants are vertically grown on 1/2 MS medium, LR formation may heavily depend on developmental signals derived from the root cap (Xuan et al., 2015, 2016). In lateral rooting on medium, the acropetal auxin flux originating from the root cap (Xuan et al., 2015, 2016) probably activates the function of ARF7 and ARF19 in xylem-pole pericycle cells for LR founder cell establishment (Okushima et al., 2007), and this process does not require WOX11 (non-WOX11-mediated rooting in Fig. 8). Wounding or stress could induce AR formation from non-root organs. In adventitious rooting, shoot-derived basipetal auxin flux can accumulate in various types of cells competent for rooting, e.g. procambium and some parenchyma cells in the leaf and stem, and procambium and xylem-pole pericycle cells in the hypocotyl (Sugimoto et al., 2010; Li et al., 2012; Ahkami et al., 2013; Sukumar et al., 2013; Liu et al., 2014; Pacurar et al., 2014). Auxin can then activate WOX11 for AR founder cell establishment (Liu et al., 2014; Chen et al., 2016; Hu and Xu, 2016) (WOX11-mediated rooting in Fig. 8). Both WOX11 in adventitious rooting and ARF7/19 in lateral rooting directly upregulate LBD16 in founder cells for later primordium development (Fig. 8). Therefore, the distinction between adventitious and lateral rooting appears to be relevant mainly at the initial developmental steps, and WOX11 is a potential molecular marker to discriminate between the two types of rooting. We hypothesize that some ARF members may directly regulate WOX11 expression in AR founder cells in response to auxin signaling, as WOX11 is indicated to be a direct target of the auxin signaling pathway (Liu et al., 2014). Identification of such ARFs will improve our understanding of the missing link from auxin to WOX11. We cannot exclude the possibility that ARF7 and ARF19 are also involved in some types of AR formation, because a previous study suggested that they are involved in adventitious rooting from hypocotyls (Wilmoth et al., 2005). Currently, it is not clear whether ARF7/19 and WOX11 are totally independent in all types of AR formation. It is also not clear whether adventitious rooting from leaves, stems or hypocotyls follows exactly the same pathway. Furthermore, we cannot exclude the possibility that other different mechanisms exist in the initiation of root primordium for lateral and adventitious rooting.
In natural conditions, root system formation in soil is complex and is under the control of both development signals and environmental cues such as nutrition, wounding and stress [e.g. root tip wounding, Fig. 3; and drought, Fig. 5 (Verslues et al., 2006)]. In this study, we show that the Arabidopsis primary root has the competence to initiate both WOX11-mediated roots and non-WOX11-mediated roots (i.e. ARF7/19-mediated LRs) when plants are grown in soil or when the primary root is damaged (Fig. 8). Primary root branching through both WOX11-mediated and non-WOX11-mediated root formation pathways in soil may represent the co-occurrence of two induction mechanisms that allow integration of developmental signals with environmental cues to optimize plant growth.
We observed that the auxin response is different in the primary roots of plants grown in soil and vertically on 1/2 MS medium, suggesting that different environmental cues may cause different auxin responses in roots (Fig. S12). A higher auxin level in soil (Fig. S12) is likely to lead to higher WOX11 expression level in soil. Analysis using BAR-Tools (http://www.bar.utoronto.ca/) (Winter et al., 2007; Geng et al., 2013) suggests that NaCl treatment can upregulate WOX11 expression, indicating that salt conditions may have a role in regulation of root system formation. In this study, the soil condition comprises many nutrients. Nutrients and many environmental signals in soil might also alter the root system (Macgregor et al., 2008; Atkinson et al., 2014; Kellermeier et al., 2014; Otvos and Benkova, 2017). In addition, LBD16 expression could be induced in the primary root of arf7-1 arf19-1 in either drought conditions or upon wounding on medium (Fig. S13). The arf7-1 arf19-1 double mutant and the WOX11 and LBD16 marker lines could provide an experimental system for the effects of inductive cues on root branching. Future analysis of the inductive cues will provide a more comprehensive understanding of how root system architecture is established and how developmental plasticity is employed in an effort to optimize response to a variable environment.
MATERIALS AND METHODS
Plant materials
The lbd16-2 (Fan et al., 2012) mutant, the wox11-2 wox12-1 double mutant (Liu et al., 2014), the arf7-1 arf19-1 mutant (Okushima et al., 2007), and WOX11pro:GUS (Liu et al., 2014) and DR5pro:GUS (Ulmasov et al., 1997) transgenic plants were described previously. For construction of LBD16pro:LBD16-GUS and mLBD16pro:LBD16-GUS transgenic plants, the 4.8-kb wild-type or mutated genomic region of LBD16 was inserted into the pBI101 vector, respectively. 35Spro:3×FLAG-WOX11-GR was constructed by insertion of the cDNA encoding the fused 3×FLAG-WOX11-GR protein into the pMON530 vector. To construct pER8:3×FLAG-LBD16, a cDNA encoding the fused 3×FLAG-LBD16 protein was PCR amplified and inserted into the pER8 vector (Zuo et al., 2000). WOX11pro:WOX11 was constructed by insertion of the WOX11 CDS following the 4.8-kb WOX11 promoter (Liu et al., 2014) into the modified pBI101 vector in which the GUS gene had been removed. GATA23pro:GATA23-GUS was constructed by insertion of the 1.6-kb genomic region including the promoter and the gene body of GATA23 into the pBI101 vector. Transgenic plants were obtained by Agrobacterium tumefaciens-mediated transformation into wild-type Arabidopsis Col-0. Primers used in molecular cloning are listed in Table S1.
Plant growth conditions
The medium conditions (1/2 MS and B5) were described previously (Chen et al., 2014; Liu et al., 2014). The soil is a 1:1:3 mixture of vermiculite: perlite: peat soil containing 1/2 MS liquid solution without sucrose. Plants were grown in soil in 20-cm-high pots. All plants and explants were grown and cultured at 22°C.
GUS staining and imaging
GUS staining was carried out based on our protocol (He et al., 2012; Chen et al., 2014). Microscopy and differential interference contrast (DIC) observations were performed as previously described (Chen et al., 2014; Liu et al., 2014) using a Nikon Eclipse 80i microscope.
ChIP and qRT-PCR
ChIP and qRT-PCR were performed as previously described (He et al., 2012). The qRT-PCR results are presented as relative transcript levels, which were normalized against that of ACTIN genes. Anti-FLAG antibody (F1804, Sigma) was used in the ChIP analysis. The primers used for real-time PCR are listed in Table S1.
Acknowledgements
We thank T. J. Guilfoyle and ABRC for Arabidopsis seeds used in this work.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.S., L.X.; Validation: L.S., X.H.; Formal analysis: L.S., X.H.; Investigation: L.S., X.H.; Resources: G.Z.; Writing - original draft: Y.D., H.H., B.S., L.X.; Supervision: L.X.; Funding acquisition: L.X.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31422005), the National Basic Research Program of China (973 Program) in association with the Ministry of Science and Technology of the People's Republic of China (2014CB943500), the Key Research Program of the Chinese Academy of Sciences (CAS) (QYZDB-SSW-SMC010), the Strategic Priority Research Program ‘Molecular Mechanism of Plant Growth and Development’ of CAS (XDPB0403), and Youth Innovation Promotion Association CAS (2014241). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.152132.supplemental
References
- Ahkami A. H., Melzer M., Ghaffari M. R., Pollmann S., Ghorbani Javid M., Shahinnia F., Hajirezaei M. R. and Druege U. (2013). Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238, 499-517. 10.1007/s00425-013-1907-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T. and Chua N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11, 605-612. 10.1046/j.1365-313X.1997.11030605.x [DOI] [PubMed] [Google Scholar]
- Atkinson J. A., Rasmussen A., Traini R., Voss U., Sturrock C., Mooney S. J., Wells D. M. and Bennett M. J. (2014). Branching out in roots: uncovering form. function, and regulation. Plant Physiol. 166, 538-550. 10.1104/pp.114.245423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C., Pacurar D. I. and Perrone I. (2014). Adventitious roots and lateral roots: similarities and differences. Annu. Rev. Plant Biol. 65, 639-666. 10.1146/annurev-arplant-050213-035645 [DOI] [PubMed] [Google Scholar]
- Chen X., Qu Y., Sheng L., Liu J., Huang H. and Xu L. (2014). A simple method suitable to study de novo root organogenesis. Front. Plant Sci. 5, 208 10.3389/fpls.2014.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tong J., Xiao L., Ruan Y., Liu J., Zeng M., Huang H., Wang J. W. and Xu L. (2016). YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 67, 4273-4284. 10.1093/jxb/erw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet I. (2012). Lateral root initiation: one step at a time. New Phytol. 193, 867-873. 10.1111/j.1469-8137.2011.03996.x [DOI] [PubMed] [Google Scholar]
- Fan M., Xu C., Xu K. and Hu Y. (2012). LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 22, 1169-1180. 10.1038/cr.2012.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Wu R., Wee C. W., Xie F., Wei X., Chan P. M. Y., Tham C., Duan L. and Dinneny J. R. (2013). A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25, 2132-2154. 10.1105/tpc.113.112896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T., Joi S., Mimura T. and Fukaki H. (2012). The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139, 883-893. 10.1242/dev.071928 [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Bussell J. D., Pacurar D. I., Schwambach J., Pacurar M. and Bellini C. (2009). Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21, 3119-3132. 10.1105/tpc.108.064758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Chen X., Huang H. and Xu L. (2012). Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genet. 8, e1002911 10.1371/journal.pgen.1002911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. and Xu L. (2016). Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 172, 2363-2373. 10.1104/pp.16.01067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeier F., Armengaud P., Seditas T. J., Danku J., Salt D. E. and Amtmann A. (2014). Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell 26, 1480-1496. 10.1105/tpc.113.122101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M. (2013). Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. J. Exp. Bot. 64, 2609-2617. 10.1093/jxb/ert155 [DOI] [PubMed] [Google Scholar]
- Lavenus J., Goh T., Roberts I., Guyomarc'h S., Lucas M., De Smet I., Fukaki H., Beeckman T., Bennett M. and Laplaze L. (2013). Lateral root development in Arabidopsis: fifty shades of auxin, Trends Plant Sci. 18, 450-458. 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Lee H. W., Kim N. Y., Lee D. J. and Kim J. (2009). LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151, 1377-1389. 10.1104/pp.109.143685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A., To J. P. C., Busch W., Stehling S., Kehle A., Demar M., Kieber J. J. and Lohmann J. U. (2005). WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438, 1172-1175. 10.1038/nature04270 [DOI] [PubMed] [Google Scholar]
- Li Y. H., Zou M. H., Feng B. H., Huang X., Zhang Z. and Sun G. M. (2012). Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol. Biochem. 55, 33-42. 10.1016/j.plaphy.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Liu J., Sheng L., Xu Y., Li J., Yang Z., Huang H. and Xu L. (2014). WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 26, 1081-1093. 10.1105/tpc.114.122887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann J. U., Hong R. L., Hobe M., Busch M. A., Parcy F., Simon R. and Weigel D. (2001). A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105, 793-803. 10.1016/S0092-8674(01)00384-1 [DOI] [PubMed] [Google Scholar]
- Macgregor D. R., Deak K. I., Ingram P. A. and Malamy J. E. (2008). Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 20, 2643-2660. 10.1105/tpc.107.055475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno M. A., Van Norman J. M., Moreno A., Zhang J., Ahnert S. E. and Benfey P. N. (2010). Oscillating gene expression determines competence for periodic Arabidopsis root branching, Science 329: 1306-1311. 10.1126/science.1191937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y., Fukaki H., Onoda M., Theologis A. and Tasaka M. (2007). ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118-130. 10.1105/tpc.106.047761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos K. and Benkova E. (2017). Spatiotemporal mechanisms of root branching. Curr. Opin. Genet. Dev. 45, 82-89. 10.1016/j.gde.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Pacurar D. I., Perrone I. and Bellini C. (2014). Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant 151, 83-96. 10.1111/ppl.12171 [DOI] [PubMed] [Google Scholar]
- Peret B., De Rybel B., Casimiro I., Benkova E., Swarup R., Laplaze L., Beeckman T. and Bennett M. J. (2009). Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399-408. 10.1016/j.tplants.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Rellan-Alvarez R., Lobet G. and Dinneny J. R. (2016). Environmental control of root system biology. Annu. Rev. Plant Biol. 67, 619-642. 10.1146/annurev-arplant-043015-111848 [DOI] [PubMed] [Google Scholar]
- Sorin C., Bussell J. D., Camus I., Ljung K., Kowalczyk M., Geiss G., McKhann H., Garcion C., Vaucheret H., Sandberg G. et al. (2005). Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17, 1343-1359. 10.1105/tpc.105.031625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Jiao Y. and Meyerowitz E. M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18, 463-471. 10.1016/j.devcel.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Sukumar P., Maloney G. S. and Muday G. K. (2013). Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiol. 162, 1392-1405. 10.1104/pp.113.217174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G. and Guilfoyle T. J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963-1971. 10.1105/tpc.9.11.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman J. M., Zhang J., Cazzonelli C. I., Pogson B. J., Harrison P. J., Bugg T. D., Chan K. X., Thompson A. J. and Benfey P. N. (2014). Periodic root branching in Arabidopsis requires synthesis of an uncharacterized carotenoid derivative, Proc. Natl. Acad. Sci. USA 111, E1300-E1309. 10.1073/pnas.1403016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer J. E. and Geldner N. (2015). Lateral root initiation in Arabidopsis thaliana: a force awakens. F1000Prime Rep. 7, 32 10.12703/P7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P. E., Agarwal M., Katiyar-Agarwal S., Zhu J. and Zhu J. K. (2006). Methods and concepts in quantifying resistance to drought. salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523-539. 10.1111/j.1365-313X.2005.02593.x [DOI] [PubMed] [Google Scholar]
- Wilmoth J. C., Wang S., Tiwari S. B., Joshi A. D., Hagen G., Guilfoyle T. J., Alonso J. M., Ecker J. R. and Reed J. W. (2005). NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43, 118-130. 10.1111/j.1365-313X.2005.02432.x [DOI] [PubMed] [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G. V. and Provart N. J. (2007). An ‘Electronic Fluorescent Pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2, e718 10.1371/journal.pone.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. and Huang H. (2014). Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 108, 1-33. 10.1016/B978-0-12-391498-9.00009-7 [DOI] [PubMed] [Google Scholar]
- Xuan W., Audenaert D., Parizot B., Moller B. K., Njo M. F., De Rybel B., De Rop G., Van Isterdael G., Mähönen A. P., Vanneste S. et al. (2015). Root cap-derived Auxin pre-patterns the longitudinal axis of the Arabidopsis root. Curr. Biol. 25, 1381-1388. 10.1016/j.cub.2015.03.046 [DOI] [PubMed] [Google Scholar]
- Xuan W., Band L. R., Kumpf R. P., Van Damme D., Parizot B., De Rop G., Opdenacker D., Moller B. K., Skorzinski N., Njo M. F. et al. (2016). Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351, 384-387. 10.1126/science.aad2776 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hu Y., Dai M., Huang L. and Zhou D.-X. (2009). The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21, 736-748. 10.1105/tpc.108.061655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Niu Q.-W. and Chua N.-H. (2000). Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265-273. 10.1046/j.1365-313x.2000.00868.x [DOI] [PubMed] [Google Scholar]