Figure 5.
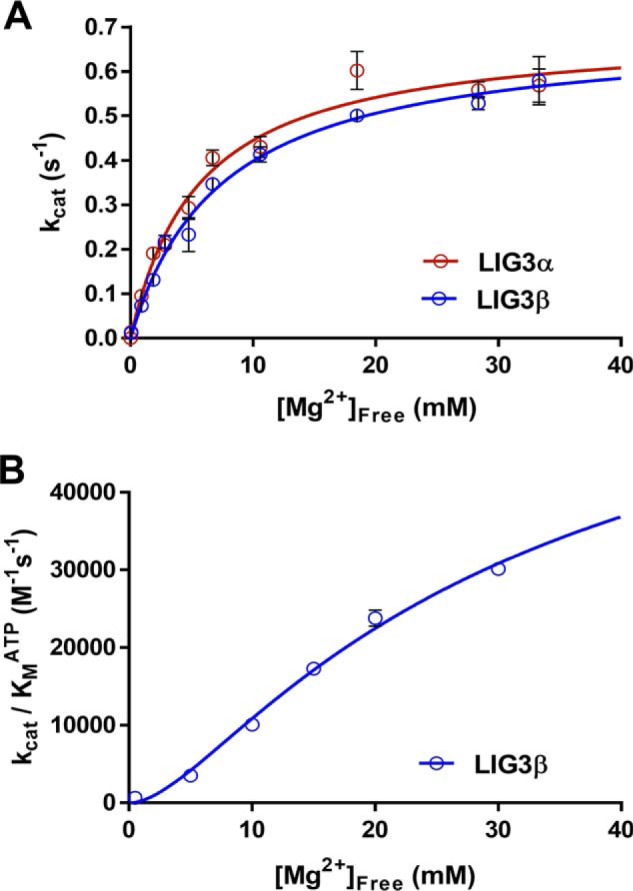
Magnesium dependence for multiple-turnover ligation. A, reactions contained 1 μm DNA, 1 mm ATP, and the concentration of free Mg2+ was varied between 0 and 35 mm. The data were fit using a hyperbolic one site-specific binding equation (Equation 2) yielding maximal kcat, Mg values of 0.69 ± 0.04 and 0.69 ± 0.03 s−1 and KMg values of 5.6 ± 0.9 and 7.4 ± 0.7 mm for LIG3α and LIG3β, respectively. B, investigation of the Mg2+ concentration dependence of enzyme adenylylation. The Mg2+ concentration dependence was performed using subsaturating ATP concentrations (supplemental Fig. S4). The magnesium concentration dependence of (kcat/Km)ATP for LIG3β was fit using a two metal random binding model (Equation 3). Each experiment was completed in at least triplicate (means ± S.D.).
