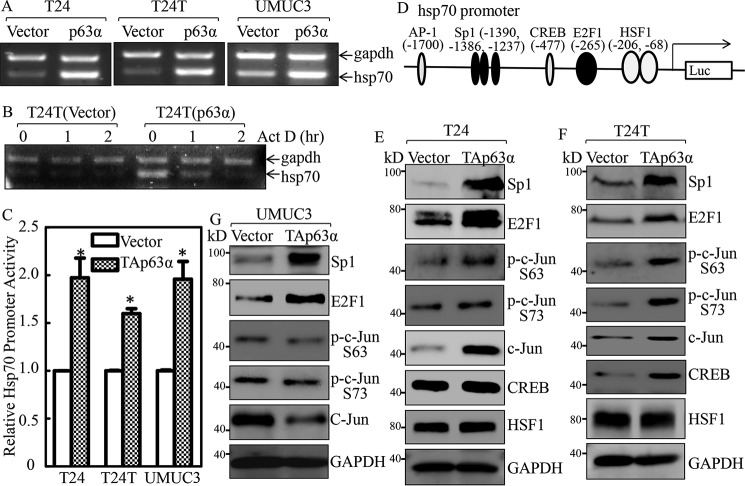
Figure 6.
Hsp70 was up-regulated at transcriptional level by p63α in bladder cancer cells. A, the indicated cells were extracted with TRIzol reagent to isolate total RNA upon the density reaching 80–90%. hsp70 mRNA levels were determined with RT-PCR by using the specific primers. GAPDH was used as an internal control. B, T24T(Vector) and T24T(p63α) cells were seeded into 6-well plates. After synchronization, T24T(Vector) and T24T(p63α) cells were treated with actinomycin D (Act D) for the indicated time points, then total RNA was isolated and subjected to RT-PCR analysis to evaluate mRNA levels of hsp70 and GAPDH. C, the indicated cells were transfected with Hsp70 promoter-driven luciferase reporter together with pRL-TK. The transfectants were seeded into 96-well plates and then subjected to determine Hsp70 promoter activity by measuring luciferase activity. pRL-TK was used as an internal control to normalize the transfection efficiency. Each bar indicates the mean ± S.D. from three replicate assays. The asterisk (*) indicates a significant increase in promoter-driven promoter activity in p63-overexpressed cells in comparison with Vector transfectants (p < 0.05). D, potential transcriptional factor-binding sites in the Hsp70 promoter region (−2000 + 1) were analyzed using the TRANSFAC 8.3 engine online. E–G, p63α stable transfectants as indicated were extracted, and the cell extracts were subjected to Western blotting to determine expression of the indicated proteins. GAPDH was used as a protein loading control.