Figure 9.
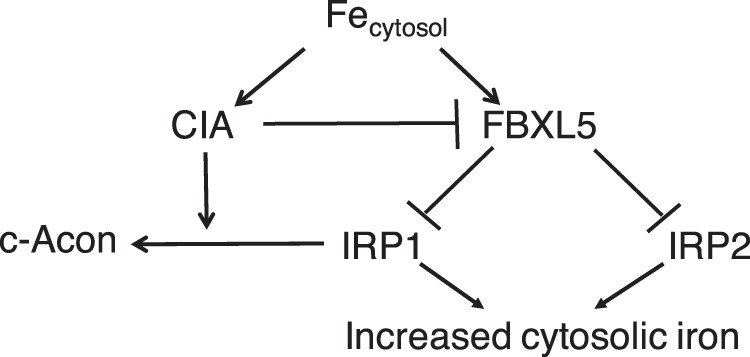
Model of regulatory processes controlling IRP1. IRP1 RNA-binding activity can be inactivated through insertion of an Fe-S cluster by the CIA pathway or by targeted polyubiquitination and degradation initiated by the ubiquitin E3 ligase FBXL5. Thus, IRP1 senses changes in cellular iron levels either through supply of iron for CIA followed by incorporation of an Fe-S cluster or by the iron-dependent activation of FBXL5, leading to degradation of the RNA-binding form of IRP1. FBXL5 protein stability is controlled by a constitutive and iron-induced mechanism (12, 13, 30). Both CIA and FBXL5 require iron, and as cells progress from an iron-deficient to an iron-replete state, inhibition of IRP1 prevents iron overload. Iron-dependent activation of these mechanisms leads to a suppression of IRP1, which reduces iron uptake. Presumably, the CIA system “acquires” iron more avidly than does FBXL5, but this remains to be determined. CIA acts to inhibit FBXL5, as evidenced by RNAi knockdown of CIA components, leading to increased expression of the ligase. The model predicts that as the CIA system increases in activity, it suppresses FBXL5, perhaps by limiting iron for formation of holo-FBXL5 or by generation of a signal indicative of increased demand for Fe-S biogenesis. In this condition, suppression of FBXL5 would enhance cellular iron uptake to meet the needs for increased Fe-S biogenesis. Once the CIA system is saturated with iron, activation of FBXL5 occurs, which then suppresses IRP1.
