Abstract
The role of steroids in human medicine is well recognized, but the major contributions made by the large domestic animals as a source of material in the discovery, isolation, and determination of the structure of the steroid hormones is less well appreciated. After a brief reminder of the early efforts to obtain a reliable source of steroids for clinical use, the narrative here is to outline one example where success was ultimately achieved for estrogen replacement therapy. Whereas knowledge of the high concentrations of estrogens in urine of pregnant women and mares dates from the late 1920s, it was not until the 1940s that the latter was shown to be a practical source. Initially, the placenta was held to be responsible, but the involvement of the fetus in each case was eventually established. The remarkable enlargement of the human fetal adrenal glands and the fetal gonads in the horse, with characteristic features of steroid secreting tissues, suggested their participation. Ultimately, it was 16-hydroxylation by the fetal liver that resulted in estriol being the major estrogen type, by far, in late human pregnancy. In the mare, the pattern of estrogen production reflected that of the growth and later regression of the fetal gonads. The characteristic production ring-B, unsaturated estrogens in the mare is derived from an alternative pathway involving retention of the additional double bond in the biosynthesis of equilin.
Keywords: feto-placental unit, estrone, equilin, fetal adrenals, fetal gonads, equine, human, steroids
Introduction
In seeking an animal model for the role of steroids in human medicine, there are few options that fulfill most requirements with the exception of some primate species. However, an interesting comparison exists—though marked differences are seen—when fetal and maternal tissues combine in a “feto-placental unit” to produce large concentrations of estrogens in urine of pregnant women and mares. The history of the establishment of this remarkable feature of human and equine pregnancies is outlined here. Before doing so, it is important to recall the major contributions made by the large domestic animals as a source of material in the discovery, isolation and determination of the structure of the steroid hormones. The primary sources of steroid hormones in all mammalian species are the gonads and adrenal glands, with the addition of the placenta in some instances.
Whereas science of endocrinology is said to have begun at the beginning of the 20th century, there had been earlier reports from both animal experimentation and human clinical observations that the gonads, in particular, were a source of “secretions” significant for reproductive functions. The hunt was on to identify the chemical nature of the agents involved, and this led to the “Golden Age” of steroid biochemistry, roughly 1930 to 1950. Biological tests were indispensable to the work of the chemists who needed large quantities of material only available from large domestic animals to provide a starting-point for isolation, purification, and identification. However, the first steroid hormone to be isolated in pure form was estrone [1-3] from the urine of pregnant women—known already as a very rich source of estrogenic compounds [4]. The structure was determined within two years of its purification, largely through the efforts of Marrian [5] and Butenandt [6]. A “second” hormone, estriol, had also been isolated in 1930 by Marrian [5]; but the principal estrogenic hormone, estradiol, eluded efforts at purification and identification until Doisy’s group [7] succeeded in 1936, starting with over 1,000 kg of pig ovaries.
Progesterone was isolated in pure form and its structure reported independently by four groups of investigators in 1934 [8-11]. Each started with extractions from over 500 kg of pig ovaries, where the ovary has several corpora lutea. Completing the contribution of domestic animals to the identification of the primary sex hormones, the isolation of testosterone from 100 kg of bull testicular tissue was reported in 1935 by Laqueur [12].
Similar work was undertaken to identify the life-maintaining activity of extracts of adrenal glands during the late 1930s and early 1940s using large batches of pig, sheep, and beef glands. Four groups of scientists were chiefly committed—Reichstein (Zurich and Basel), Kendall (Mayo Clinic), Wintersteiner (Columbia and Squibb), and Cartland (Upjohn) in which about 30 different steroids were isolated, including six active ones. This “heroic” work was described in 1943 by Reichstein and Shoppee [13]. Its scale is difficult to imagine today but is best illustrated from Reichstein’s account: 20,000 cattle gave 1,000 kg glands, from which 900 g of crude concentrates were obtained; further fractionation yielded 26 g of material containing all of the biological activity. Cortisol was seen as the most active steroid involved in carbohydrate metabolism and is the major glucocorticosteroid secreted by the human adrenal cortex.
Other steroids in human adrenal venous blood are detected in much smaller amounts— particularly aldosterone, the most potent mineralocorticoid. A disproportionately high level of activity in survival tests in adrenalectomized animals remained in the “amorphous fraction” left after crystallization of the known compounds in adrenal extracts. This activity was related to mineral metabolism rather than being a glucocorticosteroid; the unknown compound was initially named electrocortin [14]. The fascinating personal history of the collaboration between Sylvia and James Tait in London and Tadeus Reichstein in Switzerland during the isolation and elucidation of the structure of electrocortin (later named aldosterone), was given by the Taits [15] as a tribute to Professor Reichstein shortly after his death at the age of 99. The starting material was an impressive 1,000 kg of beef adrenals—a further example of the important role of domestic animals in the discovery of the steroid hormones.
Soon, it became clear that the supply of all the hormones needed for medical use could not be met by extracts from animal sources. When the chemical synthesis of each hormone was eventually achieved in the laboratory it served to confirm the identity and structure of each steroid and led to the replacement of animal tissues as source of steroids for medical use. A notable exception was the “harvest” of estrogens from the urine of pregnant mares in the production of a commercially successful drug, Premarin. It has remained prominent in hormone replacement therapy from its first clinical introduction by Ayerst, McKenna, and Harrison, in Canada in 1941 and the United States in 1942—as exemplified by citing just one [16] of the four papers on Premarin in the same issue of a journal in 1943.
Biosynthesis of Estrogens in Pregnancy
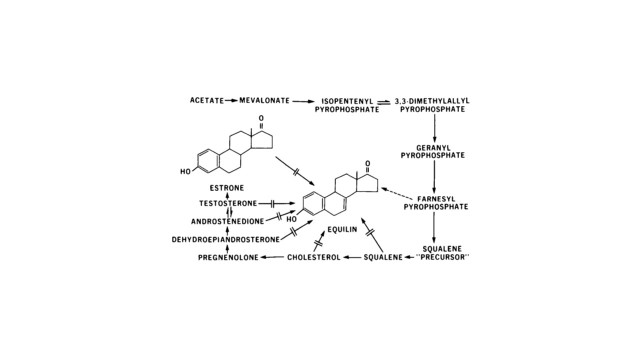
Amongst eutherian mammals, there are two species that are remarkable for estrogen production in pregnancy; the human (Homo sapiens) and the horse (Equus caballus). Other higher primates and equids have received much less attention although estrogens levels are known to rise in pregnancy. Except for a few domestic animals, little has been done to measure hormones of pregnancy in the large number of other eutherian animals (≈4000). The placenta was thought to be the site of origin of the large quantities of estrogens found in urine of pregnant women until as late as 1953 [17], which was based largely on the prompt cessation of estrogen excretion after delivery of the placenta. However, a more complicated process for estrogen production emerged from subsequent studies that involved the fetus. Brief accounts of the investigations that led to the establishment of a feto-placental unit for estrogen production in human and in equine pregnancies are the subject here. Marked differences are revealed between them— in the tissues involved, products formed (Figure 1) and patterns of urinary excretion (Figure 2 and Figure 3a,b). The salient point is that estriol is seen only in human urine and equilin only in pregnant mares’ urine.
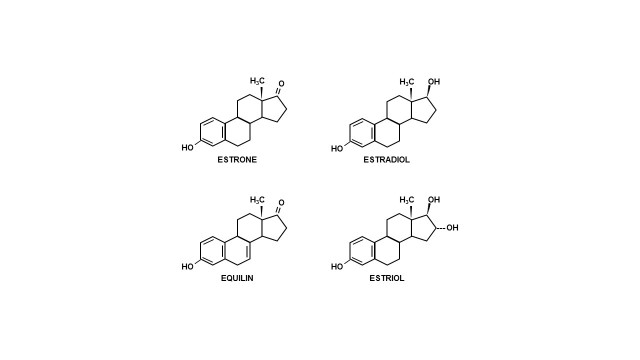
Figure 1.
Structure of estrogens formed by the placenta.
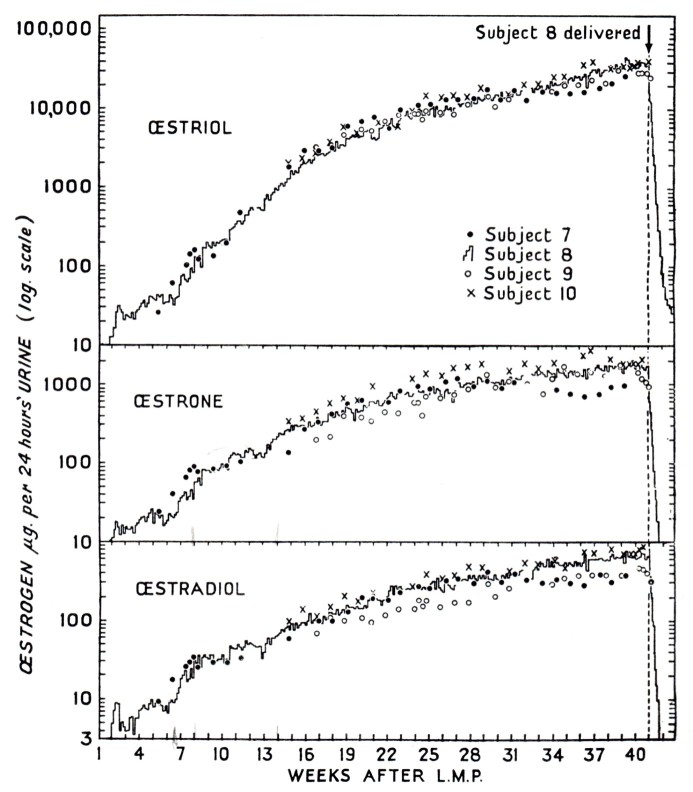
Figure 2.
Urinary excretion of estrogens during normal pregnancy in four human subjects. (From Brown, 1956 [63]).
Figure 3.
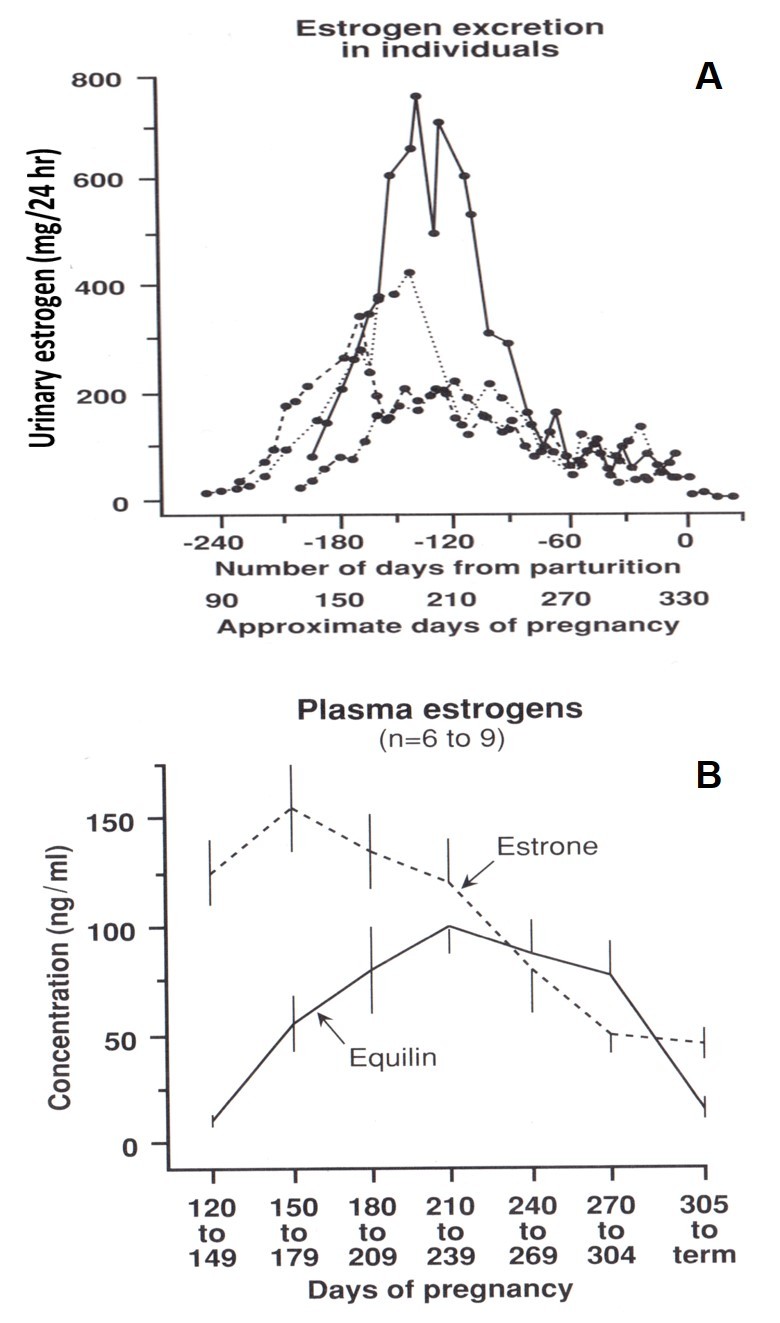
a. Patterns of total urinary estrogen excretion for four pregnant pony mares. (Ginther, 1992; adapted from Raeside and Liptrap, 1975 [64]). b. Mean plasma estrone and equilin concentrations in four pregnant mares. (Ginther, 1992; adapted from Cox, 1975 [64]).
Human Feto-placental Unit
Estrogenic activity of placental extracts was first demonstrated by Fellner in 1912 [18] and repeatedly thereafter by others. Isolation efforts were made subsequently in several laboratories which showed that estrogenic activity in human placental extracts was due to the presence of at least three compounds: estriol, estrone, and 17β-estradiol. Estriol was the major component, as had been established in the earlier work on urine from pregnant women. Later, the amounts of the “classical estrogens” present in the full-term placenta were estimated chemically in approximate amounts: 140 μg estriol, 35μg estrone, and 38μg estradiol in one kilogram of tissue [19]. The corresponding quantities for the same estrogens in a 24-hour specimen of human pregnancy urine were 10 to 30 mg, 1 to 3 mg, and 0 to 0.6 mg, respectively. In addition, it had already been reported by Collip in 1930, that estrogens were also present as water-soluble conjugates (“Emmenin”) in human placental extracts [20]. Many other products of endocrine interest were isolated from the placental collections at that time but are not relevant to an account on the biosynthesis of estrogens.
Since the placenta had large amounts of estrogens, it was reasonable to assume that the fetus would be exposed to high concentrations. Early reports suggested this to be the case when higher amounts were found in fetal liver than in placental tissue, based on biological assays [21,22]. Confirmation by chemical measurements came later when concentrations of estriol exceeded those of estrone and estradiol combined, and were also greater in the fetal liver [17]. Because it had recently been shown that incubation of estradiol with adult human liver slices led to formation of estriol, there was a strong likelihood that the fetal organs may actively participate in metabolism of estrogens. Although this was far short of evidence for participation in actual production, the possibility of a role for fetal glands presented an interesting challenge. Nonetheless, high amounts of estrogens in fetal adrenal glands of stillborn infants led Professor Jayle in Paris to propose, in 1951, that fetal adrenals represent a source of the estrogens formed in the later stages of pregnancy [23]. In fact, the concentrations in the adrenal glands were higher than found in the placenta. Careful preparation for bioassay of estrogenic activity revealed high levels in liver and adrenals but very low in kidneys and heart. The difference in the levels between adrenal and kidney (about 15-fold) spoke strongly against simple uptake by the adrenal—also noted was the close anatomical and embryological relationships of these organs. This report contained interesting unpublished data that strengthened the case for a fetal contribution to estrogen production in pregnancy. They had found large amounts of a derivative of the intermediary metabolism of the “follicle hormone, estrin”, probably a ketosteroid, which represented a step between the “follicle hormone” and estriol. The compounds detected in Jayle’s work were most likely 16α-hydroxy-estrone and 16-keto-estradiol, which were not identified in human pregnancy urine until 1957—in Marrian’s laboratory in Edinburgh [24,25]. Not until the 1960s were these same compounds isolated from the urine of newborn infants and as metabolites of estrone and estradiol in the fetus.
Support for adrenal involvement came later from an exceptional opportunity to study the local synthesis of cholesterol in the adrenal glands of an anencephalic infant. Absence of the adrenal cortex in anencephaly had been observed at least from the 1700s, but interest in the human fetal adrenal gland beyond its morphology did not seem to have been awakened until around the 1950s. In 1956, Davis and Plotz [26] described their findings in a key experiment in which the adrenals of an anencephalic infant did not synthesize— at least to an appreciable extent—radiolabeled cholesterol from the precursor 14C-acetate. The following quotation from their review gives a strong indication of the direction of thought at that time.
“The adrenal gland of the developing fetus is characterized by a thin outer zone which becomes the adrenal cortex of the individual after birth. The thick inner zone, the so-called ‘androgenic zone’, ‘X-zone’, or ‘provisional zone’, grows rapidly during the last three months of intra-uterine life and degenerates after birth. The provisional zone is absent or almost absent in anencephalic infants in which the adenohypophysis is usually very small and abnormal in shape while the neurohypophysis is either absent or rudimentary.”
Although it had been suggested that the provisional zone might be androgenic tissue, subsequent work failed to reveal any biologically active androgenic material in these glands. On measuring specific androgens in maternal and cord plasma in several cases of vaginal delivery in another study at about the same time, two 17-ketosteroids were present as conjugated steroids in all cases examined. The amount of dehydroepiandrosterone (DHEA) was higher than in the maternal blood [27]. It should be noted that DHEA, as a sulfate (DHEAS), is the major steroid secreted normally by the adult adrenal glands [28]. Since newborn females had similar levels to those of newborn males it tended to eliminate the testes as the source. Although it was not possible to say with any certainty that the fetal adrenals were producing excessive amounts of the precursor of the conjugated DHEA, the evidence was strongly indicative.
The next step towards the eventual discovery of the nature of the fetal-placental unit for estrogen formation in human pregnancy came from investigations on clinical cases of anencephaly. A characteristic absence of the anterior hypophysis (pituitary gland) is the likely cause of failure in development of the adrenal glands. In 1961, Frandsen and Stakeman [29] reasoned that if the fetal adrenals participate in the production of estrogens during pregnancy it would be natural to expect anencephaly of the fetus to be associated with low estrogen excretion during the last part of pregnancy. Levels were normal for urinary 17-ketosteroids, pregnanediol and gonadotrophins but only one-tenth for the estrogens. In all cases there was a marked hypoplasia of the fetal adrenal glands due to the so-called “fetal zone” being almost absent. To test their hypothesis, spayed female mice were injected with human fetal adrenal and placental tissues from legal abortions—indication for abortion was most often psychiatric. Signs of estrogenic activity in vaginal smears taken daily were positive only after the injection of the combination of fetal adrenal and placental tissues [30]. They summarized their findings:
“From our experience on the hormone excretion in pregnancies with anencephalic foetus and from the results of the experiments described here we feel justified in concluding that most of the oestrogen excreted during human pregnancy is neither produced by the placenta nor by the foetal adrenal, but by a complex procedure involving both tissues. Perhaps the foetal adrenal produces a substance capable of accelerating a placental production, but we think it more likely that the foetal adrenal produces a steroid precursor which is metabolized to oestrogens by the placenta and excreted as such in the urine of the mother. This assumption is supported by the fact that placental tissue in vitro can metabolize several steroids to oestrogens.”
Attempts to identify a precursor were unsuccessful in experiments in which placental tissue was injected into mice together with several steroids, some of which had recently be shown to be synthesized by fetal adrenals, at least in vitro. No estrogens were formed from progesterone, 16α-hydroxyprogesterone, 17α-hydroxyprogesterone, pregnenolone, dehydroepiandrosterone, or androstenedione [31]. It is not surprising that the first four compounds, gave negative results because later evidence, from several laboratories, showed that the placenta is largely incapable of removing the 2C side-chain to convert C21 to C19 steroids. It is possible that the two androgens were metabolized by the host tissues to steroid products (e.g. 5α/5β-reduced)—that cannot be converted into estrogens—before reaching the placental tissues. Tissue cultures of fetal adrenals would have been most helpful but success in keeping them alive had eluded several laboratories in the 1950s. Nor had anyone found a suitable species to pursue work that would be comparable to that on the human fetal adrenal gland. The so-called “X-zone” of the mouse is not comparable to the human fetal zone; the X-zone degenerates only a long time after birth—not until androgens rise at puberty in the male or a pregnancy occurs in the female. Moreover, an increased urinary excretion of estrogens during pregnancy had been found in only a few animal species; none matched the situation in human pregnancy in either pattern or quantity (Figure 2). At least for quantity, the most interesting animalwas perhaps the pregnant mare which excretes considerable amounts of estrogens in the urine. The discovery, exploitation, and elucidation of estrogen biosynthesis in the pregnant mare form is the subject of second part of this review. However, before turning to that task it is necessary to address the final stages in the exposition of the feto-placental unit in pregnant women.
Against the fetal adrenal glands being a site of estrogen production, it had been shown that estrogen concentrations were lower in the adrenal than in the liver; furthermore, most of the adrenal estrogen was present as a characteristic end-product, conjugated estriol [31]. In organs concerned with making steroid hormones, the “free” form is generally predominant; thus, estriol in the fetal adrenals in the second trimester was presumed to be elaborated elsewhere—most probably in the placenta. It was the presence of estrogens as conjugates in the fetus and the marked difference in the estriol / estrone + estradiol ratio between fetal and placental tissues that posed difficulties in establishing the source of the large amounts of estrogens seen in pregnancies [32]. Clearly the fetus could metabolize estrogens but was unlikely to be the site of biosynthesis. The surprising discovery that DHEA, as a sulfoconjugated steroid (DHEAS), was the major steroid secreted by the adrenal glands in human subjects [28] proved to be the key to the conundrum.
Events moved quickly in the 1960s. In December 1963, the conversion of [3H]-DHEAS to [3H]-estrogens on intravenous injections to pregnant and non-pregnant women was reported by Baulieu, in Paris [33]. Conversion was much higher in pregnant women, signifying that the maternal DHEAS was not the unique precursor of urinary estrogens and implying that the placenta, or the fetal-placental ensemble, was the major contributor. Moreover, from the data on estriol it could be inferred that an obligatory intermediate between DHEAS and estriol must exist—perhaps a 3β, 16α-dihydroxy-∆5-steroid. The rapid pace of progress is best illustrated by a private communication from Egon Diczfalusy (Dec., 1963) to the author:
“—we just finished three manuscripts on the in vivo aromatisation of DHA and DHA sulfate in pregnant women. Our results indicate that DHAS circulating in the fetus and in the mother may well be the exclusive precursor of all urinary estrogen in pregnant women. It seems that placental estriol is formed from DHAS via a 16-hydroxylated intermediate which was shown to be formed by the fetal compartment. I will try to send you a proof copy as soon as I get one, but I am afraid that it may not be possible for you to participate in the subsequent studies on the aromatisation of 16-hydroxylated C-19 steroids, since such studies are already in progress.”
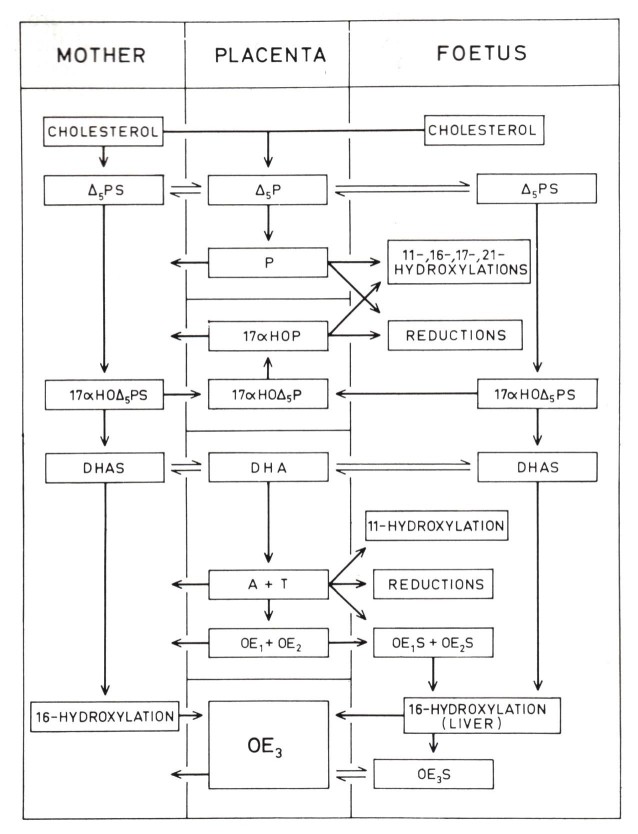
The studies referred to appeared in a back-to-back series of three papers, all submitted to the journal in December 1963, and another in 1966. Finally, there was clear evidence that DHEA and DHEAS are exposed to an extensive 16-hydroxylation, and that the fetal liver is the principal site of this reaction. Thus, in its simplest terms, the feto-placental collaboration in the production of estriol can be reduced to two essential points. First, the placental contribution is seen almost exclusively as one of aromatization, an androgen conversion to estrogen. The second point is that the fetal adrenal glands synthesize DHEAS and its 16-hydroxylation takes place in the fetal liver. A lack of a full complement of enzymes by either partner is resolved through collaboration.
These studies and the following work to investigate other aspects of steroid metabolism in pregnancy and the feto-placental unit in Diczfalusy’s laboratory in Stockholm were made possible by permission for interruption of pregnancy at about the 17th to 20th week. This was granted upon request by the patients to the Royal Medical Board of Sweden—in accordance with the statute of 1938, amended in 1948 and 1963. All these types of investigation ended in the 1970s when non-surgical termination at this stage of pregnancy became possible and, henceforth, the accepted practice. Several reviews have described the work and are interesting especially when viewed in chronological perspective [34-36]. The “final” scheme is complex, admittedly, and reflects steroid metabolism only at mid-pregnancy (Figure 4). It seems reasonably applicable, with some quantitative changes, to the last trimester when estriol synthesis is at its zenith (Figure 2).
Figure 4.
Suggested scheme of the principal pathways of steroidogenesis in the human fetoplacental unit at midpregnancy. (Diczfalusy, 1955 [35]).
Equine Feto-placental Unit
The remarkably high production of estrogens by the pregnant mare was first brought to light in 1930, by Zondek’s report [37]. Before the nature of the collaboration in human pregnancy between fetus and placenta was uncovered in the 1960s, the placenta was assumed to be responsible for the high amounts of estrogen in pregnant mares’ urine. Why the placenta, rather than the ovaries, was thought to be the source was based on the pioneering studies of H. H. Cole and his colleagues at the University of California in the early 1930s. Biological tests with plasma and urine samples from early to late pregnancy in the mare showed ovarian stimulation in immature rats initially, then high levels of estrogenic activity about mid-pregnancy with a decline towards the end [38]. Observations on the mares’ ovaries themselves were informative. Formation of several corpora lutea closely correlated with the time of high concentrations of the ovary-stimulating hormone in blood (Pregnant Mares’ Serum Gonadotrophin). They regressed from mid-gestation and were seen only as vestiges at later stages. Interestingly, one mare had a resorption of the fetus, probably beginning during the first 100 days of pregnancy; only traces of estrogen were encountered when followed until the 200th day. Clearly, the production of estrogen in large amounts depended upon the presence of a living fetus and it seemed highly improbable that the maternal ovaries ever contribute significantly.
With the fetus implicated, the following account of the work leading to the discovery of the role of the fetus is offered. A feature of pregnancy that was cause for some speculation over the years was the development of the fetal horse gonads [39]. A description of the histology of the testis of a newborn foal by Franz Leydig (1850) was part of his extensive, comparative studies of the testis, with emphasis on the interstitial cells that later would immortalize his name. He noted their relation to the vascular channels in a way that later was recognized as an ideal arrangement for an organ of internal secretion. Others later noted a resemblance between the interstitial cells of both the fetal testis and ovary and the cells of the corpus luteum. Remarkably, the ovaries of a 7- and 8-month horse fetus were much larger than those of a newborn and even larger than those of a grown horse. The California study was largely in agreement with the earlier reports and may be summarized as follows: fetal gonads of the horse undergo a remarkable growth with a rapid increase in weight between 100 and 200 days of intra-uterine life, and no differences between the sexes can be seen in this regard. By about the 7th month the fetal gonads are larger than the ovaries of the mother. The enlargement is for the most part an increase in number and size of the interstitial cells. As pointed out by Cole [39]: “Such quantities of almost pure gonadal interstitial tissue for extractive purposes cannot be found anywhere else in such abundance as in the fetal gonads of the horse.”
Tests were made for the presence of progestin, estrin, and male sex hormone in extracts of these tissues—300 grams of fetal ovaries and 300 grams of fetal testes—using biological endpoints to reach a crude quantitative assessment. For this work, more exact measures of hormone content by chemical assays were not yet at hand for any of the sex hormones. Although the possibility that the large amounts of “oestrin” excreted by the mare in the urine during the second half of pregnancy—when the maternal ovaries are quiescent—might be of fetal origin, the consensus was that the “oestrin” present in fetal tissues and mares’ urine was from the placenta. Not much academic interest in fetal horse gonads was expressed over the next twenty years or more when commercial exploitation of pregnant mares’ urine was taking place to produce Premarin as a source of estrogens for hormone replacement therapy.
The impressive development of the interstitial cells of the fetal horse gonads, with their luteal cell-like appearance, presented an opportunity to try out some of the “new” cytochemical methods that were being applied to the endocrine organs. Using this remarkable concentration of almost pure interstitial cells, histological studies with a battery of reactions—not one of them specific for steroids but strongly indicative when all reactions were positive—suggested that the fetal gonads may be a site of origin, in addition to the placenta, of the large amounts of urinary estrogens excreted by the pregnant mare [40]. Further studies with a more specific histochemical reaction for locating a site of steroid hormone production—namely, 3β-hydroxysteroid –dehydrogenase (3β-HSD) which converts ∆5 → ∆4 steroids—were negative [41]. However, light and electron microscopic studies pointed to an involvement in steroid biosynthesis [42]. To resolve this apparent impasse would require a more biochemical approach.
A report appeared in 1967 that started to open up the subject; it entailed incubation of tissues from fetal testes with 14C-acetic acid to determine the nature of the radio-labeled products formed [43]. Because 14C-acetate is a “universal” substrate the radiolabel could be found in fats, carbohydrates, and proteins whose synthesis was much greater than for steroids—by several orders of magnitude. The yield of cholesterol (0.037 percent) was by far the highest, with DHEA quantitatively the second most important steroid isolated (0.014) and estrone at only 0.0004 percent. Notwithstanding, it was concluded that DHEA might be an important precursor of estrogens in the pregnant mare. In a series of investigations at the Ontario Veterinary College in the 1970s this was later shown indeed to be the case. The first step was a time-honored approach in endocrine studies. Removal of a putative endocrine organ or gland should result in the disappearance of the product (hormone) from the blood or urine. With some trepidation, it was considered that a similar approach might lead to useful information if the fetal testes or ovaries were removed when estrogen production was high in the pregnant mare. Although no direct assessment of steroids in blood leaving the fetal gonads could be made on a continuous basis, it was deemed possible to monitor the day-to-day excretion of estrogens in the urine of the mare. In this way, it could be determined if the fetal gonads were engaged, even if the exact nature of the role remained uncertain.
This would be no simple operation and it is difficult to convey the surgical challenge in words. In initial trials, four pregnant pony mares aborted within 48 hours of fetal gonadectomy. With laparotomy alone in three mares, urinary concentrations of estrogens remained largely unchanged for one week and mares were still pregnant 4 weeks later [44]. With some modifications in surgical techniques, the effects of bilateral (n = 5) and unilateral (n = 4) fetal gonadectomy on urinary estrogen excretion were examined. Removal of both gonads resulted in a decline to about 2 percent of the pre-operative levels by 5 days. Would a single fetal gonad maintain the pregnancy? Two mares retaining pregnancies for 9 and 15 days after surgery maintained levels at 30 to 40 percent after an initial decline. While not completely satisfactory, there was enough evidence to implicate the fetal gonads in the production of the large quantities of urinary estrogens. Support subsequently came from similar attempts by others who had greater success with fetal gonadectomy [45]. Gonads were removed in four foals; estrogen concentrations in plasma fell sharply to baseline values at 24 to 48 h and remained low—three foals showed marked dysmaturity and died at birth. However, the fourth foal was delivered prematurely at 98 days after the operation and lived. In our studies, all pony mares recovered fully and after convalescence were not rebred but mostly became pets or even selected for riding schools.
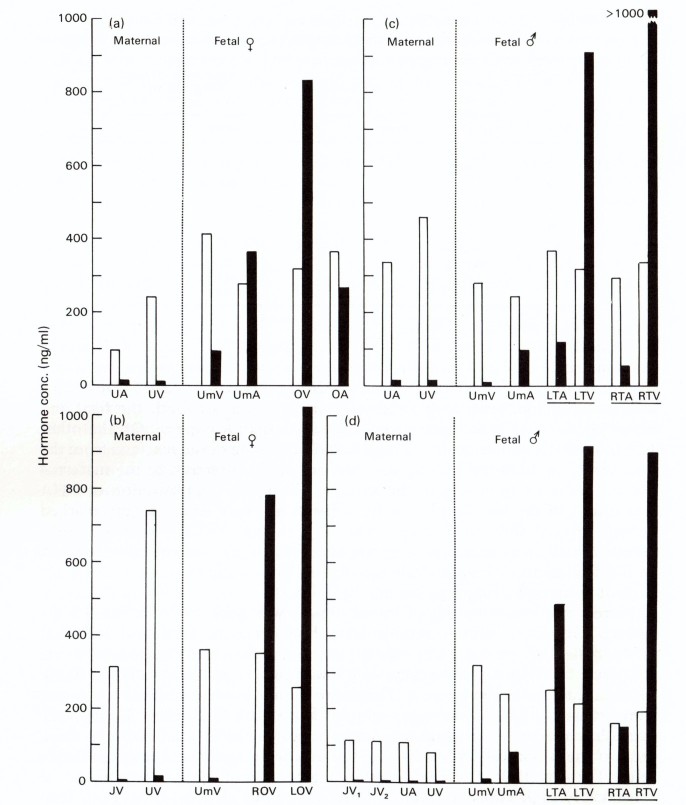
The next step was an investigation on the steroid content of the fetal gonads to find potential precursors for the formation of estrogens by the placenta. Extracts from the frozen testes and ovaries, collected in the earlier studies, were used to obtain final identification of DHEA by infrared spectroscopy [46]. Thereafter, the precursor role for DHEA was clearly demonstrated in the classical manner by measuring DHEA and estrogens in samples taken from fetal blood vessels—both gonadal and umbilical [47]. Remarkably high concentrations (> 800 ng/ml) of DHEA were found in venous samples from fetal ovaries and testes, with much lower levels in corresponding arteries (Figure 5).
Figure 5.
Plasma levels (ng/ml) of estrogens (open columns) and DHEA (solid columns) in maternal and fetal blood vessels in four mares carrying a female or male fetus. A, artery; V, vein; J, jugular; U, uterine; Um, umbilical; O, ovary; T, testis; L, left; R, right. (Raeside et al. 1979 [47]).
There remained the question of the “unique” ring B unsaturated estrogens present in large amounts in pregnant mares’ urine. Although equilin and other ring B unsaturated estrogens were first isolated in 1932 [48], their biosynthetic origin remained a mystery. In the 1950s the application of 14C to the study of metabolism of steroid hormones was introduced and offered an attack on problems hitherto unsolvable by classical methods. Administration of estrone-1-14C to the pregnant mare, with extraction and separation of estrogens from urine, showed that equilin and equilenin are not derived from estrone. Furthermore, with sodium acetate-1-14C all three were radiolabeled and the findings indicated that the ring B estrogens were formed by a different pathway [49]. Cholesterol-4-14C failed to yield radiolabeled estrone but the evidence did not rule out cholesterol of fetal origin in the biosynthesis of estrone [50].
It was generally accepted that ring B unsaturated estrogens were the “private preserve” of the pregnant mare. However, a report on the isolation of equilenin from a feminizing adrenal carcinoma of a 22-year-old male patient was startling because it had never been recorded from a human source [51]. The potential value as a diagnostic aid for detecting tumors of this type was not fulfilled in later attempts. Nevertheless, an investigation of the pathway to equilin was undertaken with specially-prepared, potential precursors (7-OH-DHEA, 7-dehydro-DHEA, 7-dehydro-androstenedione, and 7α-OH-DHEA) [52]. Each human full-term placenta was perfused with one of the selected compounds; perfusions began within 30 min of delivery and lasted for two hours after the steroid had been added to the perfusion medium. It was shown for the first time that ring B unsaturated estrogens could be formed from C19 steroids in a normal human steroidogenic organ. Although the 7α-OH compound had much lower conversion efficiency it seemed likely that the biosynthesis of ring B unsaturated estrogens from C19 steroids followed the pathway:
DHEA → 7α-hydroxy-DHEA → Δ7-DHEA → Δ4,7-‘DHEA’ → equilin → equilenin
There was one further notable attempt within the decade to address the question of a possible conversion of Δ7-C19-steroids to Δ7-estrogens. In a search for a cholesterol-lowering drug at Ayerst in Montreal, one compound (AY-9944) not only lowered blood cholesterol but did so by blocking the transformation of 7-dehydrocholesterol to cholesterol when incubated with preparations of rat liver [53]. The Ayerst group then investigated the biosynthetic origin of the Δ7-bond in equilin using a microsomal preparation of human placental tissue with three “normal” androgens (testosterone, androstenedione, and DHEA) and three androgens with a Δ7-bond as substrates. The placental preparations had the capacity to aromatize Δ4,7- and Δ5,7-C19-precursors without affecting the Δ7-bonds, but lacked the capability for introducing a Δ7-bond into estrogens or their Δ4- or Δ5-C19 precursors.
There followed a remarkable series of experiments undertaken to unravel the mystery of the biogenesis of equilin in the mare. They have been described at length by B. Bhavnani [54] as “The Saga of the Ring-B Unsaturated Equine Estrogens.” The original hypothesis had been based on the previous reports from the in vitro studies with the placenta.
At an initial test where 14C-7-dehydro-DHEAS had been injected into the jugular vein of a pregnant mare, both equilin and estrone isolated from urine were devoid of radioactivity. The sulfated form had been chosen because the steroid is present largely as a sulfate in human blood. Excessive dilution of the labeled precursor in the mare was suggested for the negative finding but no satisfactory explanation could be given. The hypothesis was discarded with the conclusion that the previous in vitro studies had little bearing on in vivo formation of equilin in the pregnant mare. As a consequence, the following experimental design was adopted. Laparotomy near term (9 to 10 months), allowed injection of mixed pairs of 14C and 3H-labeled precursors directly into the umbilical cord or intramuscularly into the fetus. Maternal urine was collected for 4 to 5 days Steroids present in the urine were isolated, purified and their radiochemical purity established. In this way, steps in the pathway of steroidogenesis from acetate to 4-14C-androstenedione were examined. In summary, the final conclusion reached was that the biosynthetic pathway to equilin and the “classical” pathway are identical until approaching the formation of the C30 hydrocarbon squalene (Figure 6). The distinctive element of the “alternative” pathway still remained elusive.
Figure 6.
In vivo pathways of equilin and estrone biosynthesis in the pregnant mare, investigated by administration of radiolabeled precursor compounds. Broken arrows indicate negative results. (Bhavnani, 1988 [54]).
Was there anything to be gained from considering the role of the fetus in the human feto-placental unit as a model? The great increase in estriol during late pregnancy in women stems mainly from high production of DHEAS, by the fetal adrenal glands, and its conversion to 16α-OH-DHEAS by the fetal liver. When delivered to the placenta, the sulfate is removed to allow aromatization to estriol. Thus, a similar scheme for the formation of equilin in the pregnant mare was investigated in the 1970s— based on the earlier reports of a precursor role for 7-OH-DHEA in human and placental tissues, in vitro. Extracts from 3H-DHEA incubations with slices of fetal horse liver yielded products with chromatographic mobility suggestive of 7-OH-DHEA. However, with preparations from human placental tissues the incubation of this material failed to show equilin formation (unpublished data).
Since DHEA in the gonadal vein of the fetus had been identified as the precursor of estrone [47], attempts were made to collect larger amounts of blood from the fetal gonads to isolate steroids in sufficient quantities for their identification. This was done by connecting a fetal gonad artery to a suitably-sized, small branch of the carotid artery in the neck of the mare and collecting blood for several minutes directly into a vessel containing heparinized saline. The second gonad was perfused with plasma substitute for 30 min in a bath of physiological saline. In each case a C19-5,7-diene was obtained—based on chromatography and characteristic UV absorbance (272, 282, and 292 nm) its identity as 7-dehydro-DHEA was then established by mass spectroscopy [55]. In an additional experiment in which dispersed cell preparations from fetal testes were incubated for 4, 6, and 8 hours a decrease in less polar products (suspected C27, C21 steroids) and an increase in the peak for 7-dehydro-DHEA were seen in extracts of the media. Meanwhile, from a series of studies by A. D. Tait [56-58], with homogenates of fetal horse gonadal tissues and radiolabeled substrates, it was demonstrated that a 5,7-diene pathway to 7-dehydro-DHEA in vitro was present. Thus, by integrating the findings of these two approaches the biosynthetic pathway to equilin was established as shown:
7-Dehydro-cholesterol → Cholesterol → → → Estrone
↓
→ 7-Dehydro-dehydroepiandrosterone → → Equilin
Clinical Significance
Hormone Replacement Therapy
Oral conjugated equine estrogens (CEE) are the most used estrogen formulation for postmenopausal hormone replacement therapy and the management of early menopausal symptoms. Since its introduction for clinical use in 1942, Premarin has been the dominant product prescribed. It contains at least ten estrogens consisting of the classical estrogens, estrone and estradiol, and a group of unique ring B unsaturated estrogens such as equilin and equilenin. Although its production from pregnant mares’ urine is a source of heated controversy (animal rights) and the potential for adverse side effects in long-term use is a matter of continued debate, it still retains a prominent position. A recent comprehensive review of the pharmacology of conjugated equine estrogens (Premarin) addressed the many questions concerning efficacy, safety, and mechanism of action [59]. Unfortunately, the earlier misconception concerning an “alternate pathway” to the biosynthesis of the ring B estrogens has been retained in the review.
Smith-Lemli-Opitz Syndrome (SLOS)
SLOS is a syndrome of multiple congenital anomalies in children first described in 1964 [60]. It is caused by a mutation in the enzyme 7-dehydrocholesterol reductase (DHCR7) [61]. Whereas elevated levels of 7-dehydro-cholesterol in tissues and amniotic fluid are fairly specific to SLOS, a non-invasive method for diagnosis would clearly be preferable. Such a method was investigated based on the studies of equilin biosynthesis in the pregnant mare [62]. Indeed, it was found that urine from a patient suspected of carrying an SLOS fetus had over half of the estrogens in the form of equine-like estriols; detection of 16α-hydroxy-17β-dihydroequilin (7-dehydroestriol) was proposed as the discriminant for non-invasive diagnosis.
Conclusion
The discovery of the way in which large amounts of estrogens are produced in pregnant women and mares has revealed a fascinating story of similarities and contrasts. While the feto-placental unit in the mare has provided an interesting subject for investigation, its importance as the principal source of estrogens for clinical use has sustained its importance for many years. However, the search for an “ideal” form of estrogen replacement therapy still remains a high priority in the advancement of women’s health and well-being.
Acknowledgments
I wish to express my appreciation for the help of Heather Christie with preparation of the manuscript and to Dr. P. Bartlewski in preparation of the figures. Dr. K. J. Betteridge provided encouragement and helpful comment on the manuscript.
Glossary
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- 7-OH-DHEA
7α-hydroxy-dehydroepiandrosterone
- ∆7-DHEA
7-dehydro-dehydroepiandrosterone
- ∆4,7 –‘DHEA’
androsta-4,7-diene-3,17-dione
References
- Butenandt A, Von Ziegner E. Uber des physiologische Wirksamkeit des krystallisierten weiblichen Sexualhormones im Allen-Doisy Test. Untersuchungen über das weibliche Sexualhormone (3. Mitteilung). Hoppe Seylers Z Physiol Chem. 1930;188:1–10. [Google Scholar]
- Doisy EA, Veler CD, Thayer S. The preparation of the crystalline ovarian hormone from the urine of pregnant women. J Biol Chem. 1930;86:499–509. [Google Scholar]
- Marrian GF. The chemistry of oestrin. III. An improved method of preparation and isolation of active crystalline material. Biochem J. 1930;24:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondek B. Hormonale schwangerschaftreaktion aus dem harn bei mensch und tier. Klin Wochenschr. 1930;9:1207–9. [Google Scholar]
- Marrian GF. The chemistry of oestrin. IV. The chemical nature of crystalline preparations. Biochem J. 1930;24:1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenandt A. Uber die chemische untersuchung der sexualhormone. Z Angew Chem. 1931;44(46):905–8. [Google Scholar]
- MacQuorquodale DW, Thayer S, Doisy EA. The isolation of the principal estrogenic substance of the liquor folliculi. J Biol Chem. 1936;115:435–49. [Google Scholar]
- Butenandt A. Neure Ergebnisse auf dem Gebiet der Sexualhormone. Wien Klin Wochenschr. 1934;47:936. [Google Scholar]
- Slotta KH, Ruschig H, Fels E. Ȕber der Hormon aus dem Corpus-luteum. Ber Chem Ges. 1934;67:1270. [Google Scholar]
- Hartmann M, Wettstein A. Ein krystallisiertes Hormon aus Corpus-luteum. Helv Chim Acta. 1934;17:878. [Google Scholar]
- Allen WM, Wintersteiner O. Crystalline progestin. Science. 1934;80:190–1. [DOI] [PubMed] [Google Scholar]
- David K, Dingemanse E, Freud J, Laqueur E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Hoppe Seylers Z Physiol Chem. 1935;233:281–3. [Google Scholar]
- Reichstein T, Shoppee CW. The Hormones of the Adrenal Cortex, in Harris, R S, Thimann, KV. Vitamins and Hormones, New York. 1943;1:345–413. [Google Scholar]
- Simpson SA, Tait JF, Bush IE. Secretion of a salt-retaining hormone by the mammalian adrenal cortex [Personal History] Lancet. 1952;260:225–8. [DOI] [PubMed] [Google Scholar]
- Tait SA, Tait JF. Personal History: the correspondence of S. A. S. Simpson and J. F. Tait with T. Reichstein during their collaborative work on the isolation and elucidation of the structure of electrocortin (later aldosterone). Steroids. 1998;63:440–53. [DOI] [PubMed] [Google Scholar]
- Freed SC, Eisin WM, Greenhill JP. The oral effectiveness of estrone sulfate (conjugated estrogens-equine) in women. J Clin Endocrinol Metab. 1943;3(2):89–91. [Google Scholar]
- Diczfalusy E. Chorionic gonadotrophin and oestrogens in the human placenta. Acta Endocrinol. 1953;12(4 Suppl):S19–175. [PubMed] [Google Scholar]
- Fellner OO. Experimentell erzeugte Wachstumveraenderungen am weiblichen Genitale der Kanichen. Centrl allg Path Anat. 1912;23:673-676. [Google Scholar]
- Huffman MN, Thayer SA, Doisy EA. The isolation of α-dihydrotheelin from human placenta. J Biol Chem. 1940;133:567–71. [Google Scholar]
- Campbell AD, Collip JB. On the clinical use of the ovarian stimulating hormone of the placenta: preliminary report. Can Med Assoc J. 1930;22:219–20. [PMC free article] [PubMed] [Google Scholar]
- Allen E, Pratt JP, Doisy EA. The ovarian follicular hormone; its distribution in human genital tissues. JAMA. 1925;85(6):399–405. [Google Scholar]
- Allen E, Doisy EA. Ovarian and placental hormones. Physiol Rev. 1927;7(4):600–50. [Google Scholar]
- Lelong M, Vandel S, Borniche P, Jayle MF. Determination of estrogens in placenta and organs of still-born infants. Ann Endocrinol (Paris). 1951;12(5):922–5. [PubMed] [Google Scholar]
- Marrian GF, Loke KH, Watson EJ, Panattoni M. 16α-Hydroxyoestrone in the urine of pregnant women. Biochem J. 1957;66:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne DS, Marrian GF. The isolation of 16β-hydroxyoestrone and 16-oxo-oestradiol-17β from the urine of pregnant women. Biochem J. 1958;70:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Plotz EJ, Leroy GV, Gould RH, Werbin H. Hormones in human reproduction. Part I: metabolism of progesterone. Am J Obstet Gynecol. 1956;72:740–55. [DOI] [PubMed] [Google Scholar]
- Nichols J, Lescure OL, Migeon CJ. Levels of 17-hydroxycorticosteroids and 17-ketosteroids in maternal and cord blood in term anencephaly. J Clin Endocrinol Metab. 1958;18(4):444–52. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Three sulfate esters of 17-ketosteroids in the plasma of normal subjects after administration of ACTH. J Clin Endocrinol Metab. 1960;20(6):900–4. [DOI] [PubMed] [Google Scholar]
- Frandsen VA, Stakemann G. The site of production of oestrogenic hormones in human pregnancy. Hormone excretion in pregnancy with anencephalic foetus. Acta Endocrinol (Copenh). 1961;38:383–91. [DOI] [PubMed] [Google Scholar]
- Frandsen VA, Stakemann G. The site or production of oestrogenic hormones in human pregnancy. II. Experimental investigations on the role of the foetal adrenal. Acta Endocrinol (Copenh). 1963;43:184–94. [DOI] [PubMed] [Google Scholar]
- Frandsen VA, Stakemann G. The site or production of oestrogenic hormones in human pregnancy. III. Experimental investigations on the role of the foetal adrenal. Acta Endocrinol (Copenh). 1964;47(2):265–72. [DOI] [PubMed] [Google Scholar]
- Diczfalusy E, Cassmer O, Alonso C, Montserrat M. Estrogen metabolism in the human fetus and newborn. Recent Prog Horm Res. 1961;17:147–99. [PubMed] [Google Scholar]
- Baulieu EE, Dray F. Conversion of H3-Dehydroisoandrosterone (3β-Hydroxy-Δ5-androsten-17-one) Sulfate to H3-Estrogens in Normal Pregnant Women. J Clin Endocrinol Metab. 1963;23(12):1298–301. [DOI] [PubMed] [Google Scholar]
- Diczfalusy E. Endocrine functions of the human fetoplacental unit. Fed Proc. 1964;23(4):791–8. [PubMed] [Google Scholar]
- Diczfalusy E. Steroid metabolism in pregnancy and the foeto-placental unit. Proc 2nd Inter Congr Horm Steroids, Exc Med Int Congr Ser. 1966;111:82-95. [Google Scholar]
- Telegdy G, Diczfalusy E. Steroid metabolism in human foeto-placental unit at mid-gestation. Proc 3rd Inter Congr Horm Steroids, Exc Med Int Congr. 1971:496-503. [Google Scholar]
- Zondek B. Hormonale schwangerschaftsreaktion auf dem harn bei mensch und tier. Klin Wochenschr. 1930;9:2285–9. [Google Scholar]
- Cole HH, Hart GH. The potency of blood serum in mares in progressive stages of pregnancy in effecting the sexual maturity of the immature rat. Am J Physiol. 1930;93:57–68. [Google Scholar]
- Cole HH, Hart GH, Lyons WR, Catchpole HH. The development and hormonal content of fetal horse gonads. Anat Rec. 1933;56:275–93. [Google Scholar]
- Davies J, Dempsey EW, Wislocki GB. Histochemical observations on the fetal ovary and testis of the horse. J Histochem Cytochem. 1957;5:584–90. [DOI] [PubMed] [Google Scholar]
- Flood PF, Marrable AW. A histochemical study of steroid metabolism in the equine fetus and placenta. J Reprod Fertil. 1975; Suppl 23:569–73. [PubMed] [Google Scholar]
- Gonzalez-Angelo A, Hernandez-Jauregut P, Martinez-Zedilo G. Fine structure of the gonads of the horse and its functional implications. J Reprod Fertil. 1975; Suppl 23:563–7. [PubMed] [Google Scholar]
- MacArthur E, Short RV, O’Donnell VJ. Formation of steroids by the equine foetal testis. J Endocr. 1967;38:331–6. [DOI] [PubMed] [Google Scholar]
- Raeside JI, Liptrap RM, Milne FJ. Relationship of fetal gonads to urinary estrogen excretion by the pregnant mare. Am J Vet Res. 1973;34:843–5. [PubMed] [Google Scholar]
- Pashen RI, Allen WR. The role of the fetal gonads and placenta in steroid production, maintenance of pregnancy and parturition in the mare. J Reprod Fertil. 1979; Suppl 27:499–509. [PubMed] [Google Scholar]
- Raeside JI. Dehydroepiandrosterone in the fetal gonads of the horse. J Reprod Fertil. 1976;46(2):423–5. [DOI] [PubMed] [Google Scholar]
- Raeside JI, Liptrap RM, McDonell WN, Milne FJ. A precursor role for DHA in a fetoplacental unit for estrogen formation in the mare. 1979. J Reprod Fertil. 1979; Suppl 27:493–7. [PubMed] [Google Scholar]
- Girard A, Sandulesco G. Sur une nouvelle série de réactifs du groupe carbonyle, leur utilisation à l’extraction des substances cétoniques et à la caractérisation microchimique des aldéhydes et cétones. Helv Chim Acta. 1936;19:1095–107. [Google Scholar]
- Heard RD, Jacobs R, O’Donnell V, Peron FG, Saffran JC, Solomon SS, et al. The application of C14 to the study of the metabolism of the sterols and steroid hormones. Recent Prog Horm Res. 1954;9:383–410. [Google Scholar]
- Heard RD, O’Donnell VJ. Biogenesis of the estrogens: the failure of cholesterol-4-C14 to give rise to estrone in the pregnant mare. Endocrinology. 1954;54(2):209–15. [DOI] [PubMed] [Google Scholar]
- Salhanick HA, Berliner DL. Isolation of steroids from a feminizing adrenal carcinoma. J Biol Chem. 1957;227:583–90. [PubMed] [Google Scholar]
- Starka L, Breuer H, Cedard L. Biosynthesis of equilin and related ring B unsaturated oestrogens in perfused human placenta. J Endocr. 1966;34:447–56. [DOI] [PubMed] [Google Scholar]
- Chappel C, Dubuc J, Dvornik M, Givner M, Humber M, Kraml K, et al. An inhibitor of cholesterol biosynthesis. Nature. 1964;201:497–8. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. The saga of the ring B unsaturated equine estrogens. Endocr Rev. 1988;9(4):396–416. [DOI] [PubMed] [Google Scholar]
- Raeside JI, Renaud RL. Identification of 3β-hydroxy-5,7-androstadien-17-one as a secretory product of the fetal horse gonad in vivo and in vitro. J Endocr. 1985;107:415–9. [DOI] [PubMed] [Google Scholar]
- Tait AD, Hodge LC, Allen WR. Production of an equilin precursor by the fetal horse gonad. IRCS Med Sci. 1982;10:346–7. [Google Scholar]
- Tait AD, Santikarn S, Allen WR. Identification of 3β-hydroxy-5,7-pregnadien-20-one and 3β-hydroxy-5,7-androstadien-17-one. J Endocr. 1983;99:87–92. [DOI] [PubMed] [Google Scholar]
- Tait AD, Hodge LC, Allen WR. The biosynthesis of 3β-hydroxy-5,7-androstadien-17-one by the horse fetal gonad. FEBS Lett. 1985;182:107–10. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR, Stanczyko FZ. Pharmacology of conjugated equine estrogens: Efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol. 2014;142:16–29. [DOI] [PubMed] [Google Scholar]
- Smith DW, Lemli L, Opitz JM. A newly recognized syndrome of multiple congenital anomalies. J Pedod. 1964;64:210217. [DOI] [PubMed] [Google Scholar]
- Correa-Cerro LS, Porter FD. 3β-Hydroxysterol ∆7-reductase and the Smith-Lemli-Opitz syndrome. Mol Genet Metab. 2005;84(2):112–26. [DOI] [PubMed] [Google Scholar]
- Shackleton CH, Roitman E, Kratz LE, Kelley RI. Equine type estrogens produced by a pregnant woman carrying a Smith–Lemli–Opitz syndrome fetus. J Clin Endocrinol Metab. 1999;84(3):1157–1157. [DOI] [PubMed] [Google Scholar]
- Brown JB. Urinary Excretion of Oestregens During Pregnancy, Lactation, and the Re-establishment of Menstruation. Lancet. 1956;267(6295):704–7. [DOI] [PubMed] [Google Scholar]
- Ginther OJ. Reproductive Biology of the Mare. 2nd ed. Cross Plains (WI): Equiservices Publishing; 1992. [Google Scholar]