Abstract
Cutaneous squamous cell carcinoma (cSCC) currently affects over 700 000 patients per year in the USA alone, and its incidence continues to rise in recent years. A known risk factor for cSCC is chronic inflammation; a cSCC that develops at a site of chronic inflammation is known as Marjolin’s ulcer. We present the case of a 76-year-old man with end-stage renal disease requiring chronic haemodialysis who developed an invasive cSCC at the cannulation site of an underlying arteriovenous (AV) fistula. In this instance, treatment with standard surgical excision or Mohs surgery would pose unique risks associated with injury to an otherwise functional AV fistula. Thus, the lesion was treated with electron beam radiation therapy, which offers a similar efficacy to surgery while minimising risk to the fistula. This resulted in a successful oncological outcome with no complications.
Keywords: Dermatology, Skin cancer, Vascular surgery, Surgical oncology, Radiotherapy
Background
Cutaneous squamous cell carcinoma (cSCC) arises from malignant proliferation of epidermal keratinocytes. cSCC is the second most common skin cancer in the USA, representing about 20% of non-melanoma skin cancers.1 2 The incidence of cSCC has risen in the USA in the past 20 years, which may be related to greater sun exposure, tanning bed use, an ageing population and improved skin cancer detection.1 Risk factors for cSCC include increased age, fair skin (and other phenotypic characteristics such as light-coloured eyes or hair,) ultraviolet light exposure, immunosuppression, chronic inflammation, arsenic exposure, personal or family history of cSCC, ionising radiation, and inherited disorders such as xeroderma pigmentosum.3–8 Sites of chronic inflammation at risk for cSCC development are diverse and include scars, burns, chronic ulcers, sinus tracts, or inflammatory dermatoses such as lichen sclerosus et atrophicus.9 The term ‘Marjolin’s ulcer’ is used to describe a cSCC that develops at the site of a chronic wound or scar. This term comes from the French surgeon Jean Nicholas Marjolin, who first described such a lesion in 1828.10
Haemodialysis for patients with end-stage renal disease (ESRD) requires durable vascular access, most commonly in the form of an autogenous arteriovenous (AV) fistula or a prosthetic AV graft. Both of these access modalities require repeated cannulation through a limited area of overlying skin. Frequently, the same puncture site is used primarily or exclusively, potentially leading to the development of chronic irritation and inflammation, infection and/or aneurysm/pseudoaneurysm formation. Some patients have received kidney transplants and thus also have an increased risk of cSCC due to long-term immunosuppression. Surprisingly, there are no cases in the literature describing cSCC development at a vascular access site. We present a case in which cSCC developed at the cannulation site of a functioning AV fistula, and discuss the multidisciplinary treatment options that were considered before the lesion was ultimately treated with electron beam radiation.
Case presentation
A 76-year-old Caucasian man with a history of ESRD due to type 2 diabetes mellitus who had been on dialysis for 7 years via a left radiocephalic AV fistula presented to the surgical oncology clinic for evaluation of an invasive cSCC of the left forearm. He had a remote history of two prior cSCCs. He had noticed a 12×10 mm pink keratotic nodule at the cannulation site for his fistula 2 months prior to presentation. Given the proximity of the lesion to his AV fistula, his dermatologist performed a shave biopsy (figure 1). This biopsy demonstrated invasive cSCC.
Figure 1.
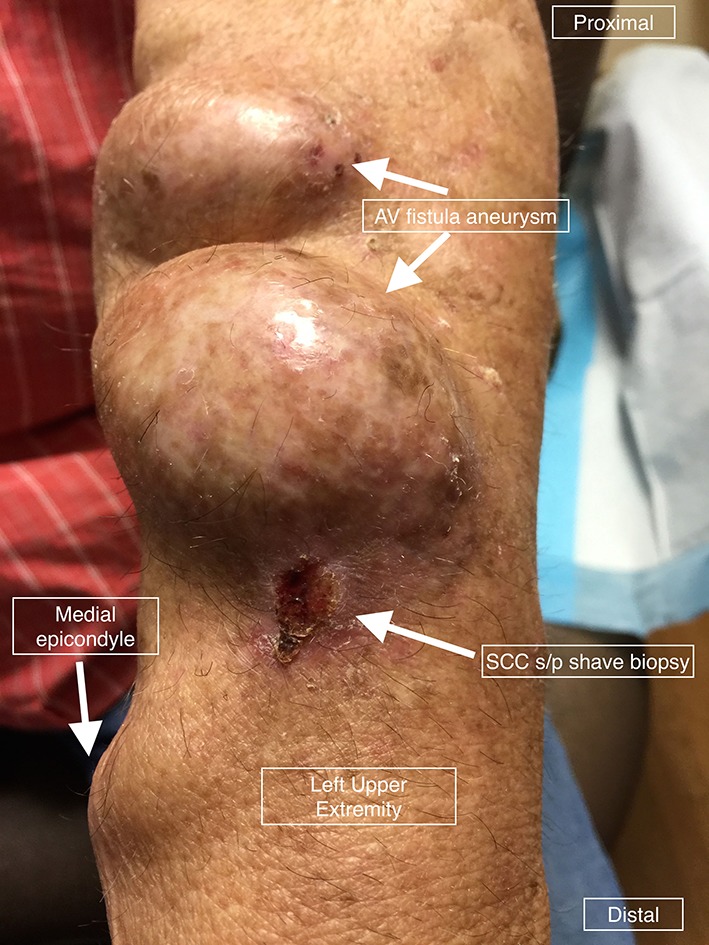
Cutaneous squamous cell carcinoma (cSCC) overlying the cannulation site of a dialysis arteriovenous (AV) fistula (status postshave biopsy at previous dermatology visit).
Treatment
Due to concern for complications related to the patient’s underlying AV fistula, the dermatologist referred the patient for treatment options. Multidisciplinary discussion among surgical oncology, vascular surgery and radiation oncology led to a decision to treat with electron beam radiation therapy. He was given a total dose of 40 Gy administered in 10 fractions.
Outcome and follow-up
The patient tolerated his full course of radiation therapy well with no complications. One month after his course of therapy, his left forearm lesion had resolved with no evidence of recurrence.
Discussion
We present what is to the best of our knowledge the first case in the literature of a cSCC at the cannulation site of an AV fistula. There is a previously described case of a cSCC in situ arising at the exit site of a previous tunnelled haemodialysis catheter in a liver transplant patient.11 While this also represents a cSCC developing at a site of chronic inflammation, that case differs in several other important regards. Specifically, the malignant lesion in that case occurred in the setting of immunosuppression and was able to be managed with surgical excision.
One limitation of this report is that the patient may have developed the cSCC at this sun-exposed site even in the absence of chronic inflammation. Given the patient’s age and history of cSCCs, development of a cSCC at this site could be coincidental.
Common therapies for invasive cSCC include surgical excision, electrodesiccation and curettage (ED&C), Mohs surgery, cryotherapy and radiation therapy.12 13 There is insufficient evidence to make conclusions about the relative efficacy of different treatments for invasive cSCC.14 15 Given this lack of evidence, the choice of treatment depends on a combination of patient and provider preferences. However, guidelines such as those published by the British Association of Dermatologists and the Scottish Intercollegiate Guidelines Network recommend surgical excision for cSCC when feasible, as this allows for histological examination of tissue margins.16 17
In determining the appropriate treatment for this patient’s cSCC, the unique risk of injury to his AV fistula, which could result in bleeding or loss of vascular access for haemodialysis, had to be carefully considered. Consequently, ED&C or Mohs surgery performed in a dermatology office setting could be accompanied by prohibitive risk. If surgical excision is undertaken, we would recommend performance of the procedure in the operating room with vascular surgery involvement or availability in the event of need for vascular repair or reconstruction. In the case presented, however, by instead using electron beam radiation therapy, the risk of AV fistula injury was minimised without compromising efficacy of treatment.
A systematic review of radiation therapy for invasive cSCC found a pooled average local recurrence rate of 6.4% among 761 patients, which was not significantly different from the recurrence rates for standard surgical excision (5.4%) or Mohs surgery (3.0%).14 In electron beam radiation therapy, a linear accelerator is used to produce a beam of electrons, which can be targeted to a tumour site. A useful characteristic of electron beam therapy is that it does not penetrate deeply, and therefore spares the tissue underlying the tumour. The energy of the electrons influences the treatment depth: in this case, 6 MeV electrons were used, which treated to a depth of 3–4 mm with a rapid dose drop-off thereafter.18 This feature was particularly useful in our case given the presence of the underlying AV fistula.
The optimal treatment of this patient’s cSCC was determined by a multidisciplinary effort involving input from a dermatologist, Mohs surgeon, surgical oncologist, vascular surgeon and radiation oncologist. Of course, not all patients with cSCC require input from such an extensive team of specialists. However, for complicated cases such as this, multidisciplinary evaluation can guide treatment decisions in order to minimise risk without compromising efficacy.
Learning points.
Cutaneous squamous cell carcinoma (cSCC) can develop over sites of chronic inflammation, including sites of cannulation for dialysis access in patients with end-stage renal disease.
The presence of an arteriovenous (AV) fistula in close proximity to a cSCC introduces an added complexity due to the concern for injury to the haemodialysis access.
Electron beam radiation therapy for cSCC is a treatment option that minimises risk to underlying structures and can be used for lesions overlying an AV fistula.
Multidisciplinary evaluation of a patient with a challenging cSCC can lead to selection of the most appropriate therapy.
Footnotes
Contributors: NSN was responsible for drafting the case report, literature review and chart review. BFG was responsible for literature review, chart review, direct care of the patient and revising the case report. RKM was responsible for advising care of the patient, describing a novel surgical technique in the report and revising the case report. PJM was responsible for conception of the case report, direct care of the patient and revising the case report. All authors have approved this version of this case report.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 2013;68:957–66. 10.1016/j.jaad.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 2. Alam M, Ratner D. Cutaneous Squamous-Cell Carcinoma. N Engl J Med Overseas Ed 2001;344:975–83. 10.1056/NEJM200103293441306 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001;63:8–18. 10.1016/S1011-1344(01)00198-1 [DOI] [PubMed] [Google Scholar]
- 4. Rosso S, Zanetti R, Martinez C, et al. . The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer 1996;73:1447–54. 10.1038/bjc.1996.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg D, Otley CC. Skin Cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002;47:1–20. 10.1067/mjd.2002.125579 [DOI] [PubMed] [Google Scholar]
- 6. Akguner M, Barutçu A, Yilmaz M, et al. . Marjolin’s ulcer and chronic burn scarring. J Wound Care 1998;7:121–2. 10.12968/jowc.1998.7.3.121 [DOI] [PubMed] [Google Scholar]
- 7. Karagas MR, Stukel TA, Morris JS, et al. . Skin Cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. Am J Epidemiol 2001;153:559–65. 10.1093/aje/153.6.559 [DOI] [PubMed] [Google Scholar]
- 8. Hussain SK, Sundquist J, Hemminki K. The effect of having an affected Parent or Sibling on Invasive and in Situ Skin Cancer risk in Sweden. J Invest Dermatol 2009;129:2142–7. 10.1038/jid.2009.31 [DOI] [PubMed] [Google Scholar]
- 9. Carlson JA, Ambros R, Malfetano J, et al. . Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol 1998;29:932–48. 10.1016/S0046-8177(98)90198-8 [DOI] [PubMed] [Google Scholar]
- 10. Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s Ulcers: Diagnosis and Treatment. J Am Col Certif Wound Spec 2011;3:60–4. 10.1016/j.jcws.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammes JS, Bestoso JT, Sharma A. Squamous cell carcinoma in situ arising at the exit site of a tunneled catheter. Am J Kidney Dis 2004;44:e43–e46. 10.1016/S0272-6386(04)00829-7 [DOI] [PubMed] [Google Scholar]
- 12. Hamouda B, Jamila Z, Najet R, et al. . Topical 5-fluorouracil to treat multiple or unresectable facial squamous cell carcinomas in xeroderma pigmentosum. J Am Acad Dermatol 2001;44:1054 10.1067/mjd.2001.113476 [DOI] [PubMed] [Google Scholar]
- 13. Morton CA, Szeimies RM, Sidoroff A, et al. . European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma. J Eur Acad Dermatol Venereol 2013;27:536–44. 10.1111/jdv.12031 [DOI] [PubMed] [Google Scholar]
- 14. Lansbury L, Bath-Hextall F, Perkins W, et al. . Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ 2013;347:f6153 10.1136/bmj.f6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Interventions for non-metastatic squamous cell carcinoma of the skin: a summarised Cochrane review. Clin Exp Dermatol 2011;36:332–3. 10.1111/j.1365-2230.2011.04056.x [DOI] [PubMed] [Google Scholar]
- 16. Motley R, Preston P, Lawrence C. Multi-professional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma 2009 [Internet]. London: British Association of Dermatologists, 2009. http://www.bad.org.uk/healthcare-professionals/clinical-standards/clinical-guidelines/scc (accessed May 2017). [Google Scholar]
- 17. Scottish Intercollegiate Guidelines Network (SIGN). Management of a primary cutaneous squamous cell carcinoma [Internet]. Edinburgh: SIGN, 2014. http://www.sign.ac.uk/pdf/SIGN140.pdf (accessed May 2017). [Google Scholar]
- 18. Petsuksiri J, Frank SJ, Garden AS, et al. . Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer 2008;112:111–8. 10.1002/cncr.23143 [DOI] [PubMed] [Google Scholar]


