Figure 1.
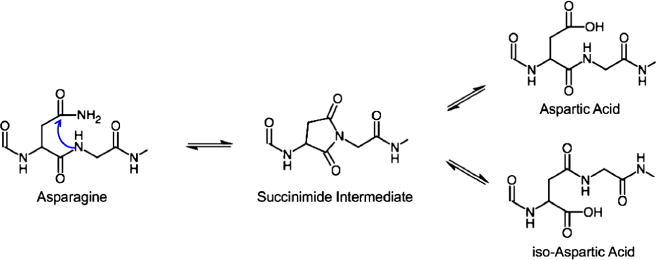
The deamidation of asparagine mechanism. Asparagine forms a five-membered succinimide ring intermediate from an intramolecular attack, and then hydrolyzes to form either aspartyl and isoaspartyl peptides (created using ChemDoodle by iChemLabs)
