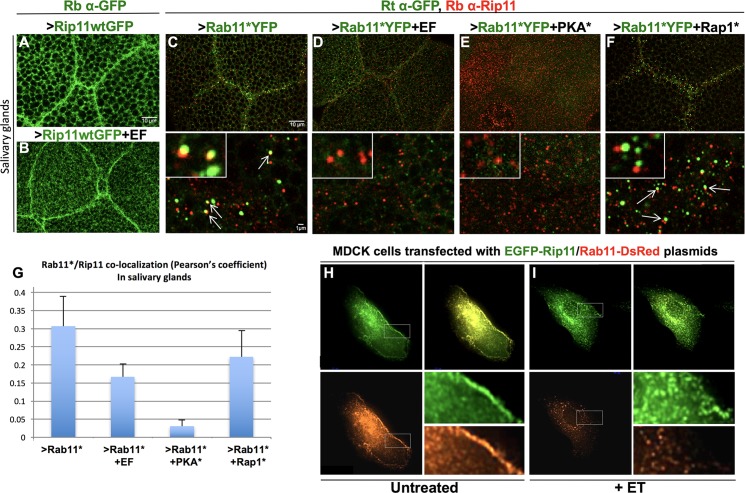
Fig 3. EF prevents association between Rab11* and its effector Rip11.
(A) Drosophila salivary glands stained with a Rabbit anti-GFP antibody (Rb α-GFP), showing that dRip11GFP accumulates at cell-cell junctions in 1096GAL4>dRip11-GFP larvae. (B) 1096GAL4>dRip11-GFP+EF glands. Junctional staining is moderately weakened by EF. (C-F) Salivary glands co-stained with a Rat anti-GFP antibody (Rt α-GFP) and a Rabbit anti-dRip11 antibody (Rb α-Rip11), showing that the co-localization between Rab11* and dRip11 detected with this antibody combination is lost or reduced upon co-expression of Rab11*YFP with EF, PKA*, or Rap1*. Lower panels show higher magnifications. Insets show representative examples of vesicles with or without Rab11*/dRip11 co-localization. (C) 1096GAL4>Rab11*YFP, arrows indicate Rab11*/dRip11 co-localization. (D) 1096GAL4>Rab11*YFP+EF. (E) 1096GAL4>Rab11*YFP+PKA*. (F) 1096GAL4>Rab11*YFP+Rap1*. Arrows point to adjacent but non-overlapping punctae. (G) Quantification of Rab11*YFP/dRip11 co-localization in panels C-F measured by the Pearson’s coefficient. (H-I) MDCK cells transfected with constructs expressing human Rip11-GFP and Rab11wt-DsRed. (H) Untreated cells showing Rab11-Rip11 co-localization throughout the cell with higher levels of Rip11 and Rab11 at cell borders. (I) Rip11 and Rab11 no longer co-localize in cells treated with ET (Edema Toxin = EF+PA). While a minor component of Rip11 is still evident at cell borders, Rab11 fails to reach the cell borders.