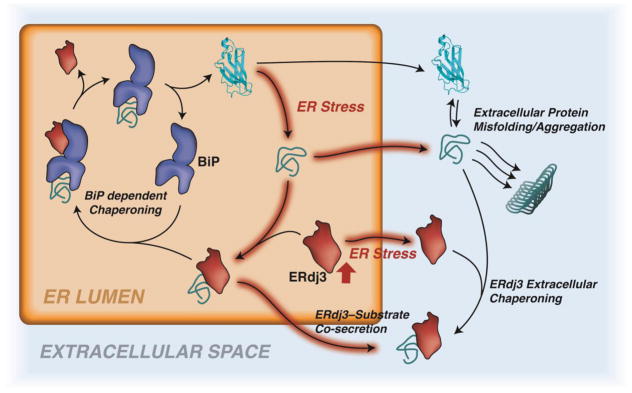
Figure 3. UPR-dependent Regulation of ERdj3 Secretion.
Illustration showing the mechanisms by which secreted ERdj3 protects the extracellular space from misfolded, aggregation prone proteins. In the ER, ERdj3 binds to misfolded protein conformations that can accumulate during ER stress. When BiP is available, ERdj3 delivers the misfolded substrate to BiP for ATP-dependent chaperoning (regular arrows). However, when BiP is limiting (due to ER stress, red-highlighted arrows), ERdj3 can be secreted when bound to non-native protein conformations, preemptively protecting the extracellular proteostasis environment from toxic aggregation-prone proteins. Alternatively, ERdj3 can be secreted in the absence of a bound protein during ER stress. This unbound ERdj3 can bind to non-native protein conformations in the extracellular space and prevent their misfolding and/or aggregation into toxic protein conformations and aggregates.