Abstract
Background
Metabolic syndrome (MS) is a well-known risk factor for the development of cardiovascular (CV) disease, yet controversy persists whether it adds incremental prognostic value in patients with established CV disease.
Objectives
This study was performed to determine if MS is associated with worse CV outcomes in patients with established CV disease treated intensively with statins.
Methods
We performed a post hoc analysis of the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) trial, in which patients with established CV disease and atherogenic dyslipidemia (n=3,414) were randomly assigned to receive extended-release niacin (ERN) or placebo during a mean 36 month follow-up, to assess whether the presence of MS or the number of MS components contributed to CV outcomes.
Results
The composite primary endpoint of CV events occurred in 15.1% of patients without MS versus 13.8%, 16.9% and 16.8 of patients with MS in the subsets with 3, 4 and 5 MS components respectively (corresponding adjusted hazard ratios 0.9, 1.1 and 1.1 relative to patients without MS), P=0.55. Comparing subgroups with 3 versus 4 or 5 MS components, there was no significant difference in either the composite primary endpoint or secondary endpoints. Patients with diabetes mellitus had higher event rates, with or without presence of MS.
Conclusions
The presence of MS was not associated with worse CV outcomes in the AIM-HIGH population. The rate of CV events in statin-treated AIM-HIGH patients with MS was not significantly influenced by the number of MS components.
Keywords: Metabolic syndrome, Cardiovascular disease, Diabetes Mellitus, Atherogenic dyslipidemia, Extended-release Niacin
Introduction
Metabolic syndrome (MS) is a cluster of biochemical and physiological abnormalities associated with an increased risk for the development of cardiovascular (CV) disease and diabetes mellitus (DM). The National Cholesterol Education Program Adult Treatment Panel III defined MS as a constellation of 3 or more components including abdominal obesity, elevated triglycerides (TG), low levels of high-density lipoprotein-cholesterol (HDL-C), elevated blood pressure, and impaired fasting glucose or insulin resistance, though it is well-recognized that there are several definitions of MS from varying professional societies. (1,2) In many of these definitions where waist circumference measurements are not obtained, a body mass index of ≥30 Kg/M2 is used as a surrogate for abdominal obesity. According to recent data from the National Health and Nutrition Examination Survey from 2003 to 2012, the overall prevalence of MS in the United States was 33%.(3)
Despite the association of MS with increased incidence of CV events, it is unclear whether MS provides prognostic value in patients with established CV disease. A recent post hoc analysis of the Clinical Outcomes Utilizing revascularization and Aggressive Drug Evaluation (COURAGE) trial demonstrated increased risk for death or myocardial infarction among patients with stable coronary artery disease who had MS at baseline; however, the MS cluster was not independently associated with increased CV events on multivariate analysis.(4)
Although niacin has been used widely in the past for the treatment of dyslipidemic patients to both increase HDL-C and lower TG levels, the most recent American College of Cardiology/American Heart Association cholesterol guidelines and American Diabetes Association position statement do not generally recommend niacin in combination with a statin to reduce atherosclerotic CV events.(5, 6) These recommendations were based on recent data from randomized placebo-controlled, secondary prevention trials that have shown no significant clinical benefit of extended-release niacin (ERN) on CV outcomes. The Second Heart Protection Study showed that ERN combined with the prostaglandin inhibitor laropiprant did not improve clinical outcomes in patients with established CV disease on statin therapy, and had an excess of significant adverse events.(7) In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial, ERN did not show clinical benefit in patients with CV disease and low baseline levels of HDL-C who were receiving statins, despite significant improvements in on-treatment HDL-C and TG levels.(8) Additionally, niacin use has been associated with an increase in fasting blood glucose and hemoglobin A1c levels in patients with DM.
The objective of the present post hoc analysis of the AIM-HIGH trial was to determine if there was predictive value of the MS constellation on the incidence of CV events in patients with established CV disease treated intensively with statins, as a function of the number of its constituent components (e.g., 3 vs. 4 or 5 MS components).
Methods
1. Study Population and Trial Design
AIM-HIGH was a randomized, controlled clinical trial designed to examine whether ERN (Niaspan™, AbbVie, Inc.) in combination with aggressive LDL-C lowering treatment could reduce the rate of CV events compared to aggressive LDL-C lowering treatment alone. Details of the design, rationale, and inclusion/exclusion criteria have been published previously.(8,9) Men and women age ≥45 years were recruited from 92 centers in the United States and Canada. Eligibility requirements included the presence of established CV disease and evidence of atherogenic dyslipidemia at baseline. Dyslipidemia criteria included low baseline levels of HDL-C (≤40 mg/dL for men and ≤50 mg/dL for women), TG levels 150 to 400 mg/dL, and low density lipoprotein-cholesterol (LDL-C) ≤180 mg/dL if participants were not taking a statin. If subjects were already receiving statin therapy at baseline, the inclusion criteria for HDL-C were ≤42 mg/dL for men and ≤53 mg/dL for women and TG levels 100 – 400 mg/dl, with the upper limit of LDL-C adjusted according to the lipid-lowering therapy and statin dose.
Prior to randomization, eligible subjects entered into a 4-to-8-week open-label, run-in phase during which they received simvastatin 40 mg per day plus ERN increasing weekly doses from 500 mg per day to 2000 mg per day as tolerated. Patients who could tolerate at least 1500 mg of ERN per day were then randomly assigned, in a 1:1 ratio, to ERN or matching placebo with 100 mg crystalline niacin (to maintain blinding) and background simvastatin therapy ranging from 40–80 mg daily.
End Points
The primary end point was the composite of the first event of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebral revascularization. Secondary composite end points included the composite of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for a “high-risk” acute coronary syndrome (characterized by accelerating ischemic symptoms or prolonged chest pain with electrocardiographic evidence of ischemia or increase in biomarker values to greater than upper limit of the normal range but less than twice the upper limit), and death from CV causes. An endpoint focusing on coronary events was constructed using time-to-first event for death from coronary heart disease, myocardial infarction, hospitalization for acute coronary syndrome, or symptom driven coronary revascularization. Upon the recommendation of the independent Data and Safety Monitoring Board, the National Heart, Lung, and Blood Institute decided to stop the trial before its planned conclusion, after mean follow up period of 3 years, based on convincing evidence of a lack of benefit of ERN on the trial primary outcome.
2. Metabolic Syndrome Categorization
Participants were categorized by the number of MS characteristics present at baseline. MS was defined according to National Cholesterol Education Program’s Adult Treatment Panel III, based on the presence of any three components: abdominal obesity, defined as waist circumference >40 inches in men and >35 inches in women, TG ≥150 mg/dL, HDL-C <40 mg/dL for men and <50 mg/dL for women, blood pressure ≥ 130/≥85 mmHg or diagnosis of hypertension, fasting glucose ≥ 110 mg /dL or presence of diabetes mellitus.(10) Diabetes mellitus was defined by a positive history of diabetes and/or taking one or more hypoglycemic medications, or baseline fasting glucose >125 mg/dL. Patients with MS were subdivided further according to the number of MS components. Data on 26 patients indicated that they had MS according to the clinical site, but the baseline examination did not confirm presence of three or more criteria for MS.
Patients were also divided into 4 subgroups by the presence (+) or absence (−) of diabetes mellitus (DM) and MS at baseline to define a risk gradient: DM−/MS −; DM−/ MS +; DM +/ MS−; DM+/MS+. The last two subgroups were combined into one group as there were only 64 patients who were DM +/ MS −.
3. Laboratory Measurements
Laboratory analyses were performed by a central core laboratory (Northwest Lipid and Diabetes Research Laboratory at the University of Washington) using standardized techniques. Fasting glucose, insulin, hemoglobin A1c, lipid profiles, including lipoprotein(a), apoprotein A, and apoprotein B, were measured at baseline. The homeostasis model assessment of insulin resistance (HOMA1-IR) was estimated using the following formula: (glucose × insulin)/405, in mass units, mg per deciliter. (11)
4. Statistical analysis
Baseline characteristics were expressed as mean +/−SD or percent and compared using analysis of variance for continuous variables or non-parametric tests depending on the distribution of the covariate, and chi square test for categorical variables. To evaluate relationship between groups without MS and MS with any number of the MS components and end point outcomes, Cox proportional hazards regression models were created adjusting for treatment group, sex, age and number of MS components. Hazard ratios (HR) and 95% confidence intervals were calculated to compare risk between the groups using the Wald test. Similarly, to assess for the relationship between the number of MS criteria and end point outcomes, similar Cox proportional hazards regression models were used. End point event rates by risk gradient were compared using Cox proportional regression models, also adjusting for treatment group, sex and age. Event rate defined as number of evens per exposure in patient-years. 95% confidence intervals were calculated based on Poisson distribution of the event rate. Time to event is displayed using Kaplan-Meier plots; groups are compared using a log-rank statistic. Analyses were done pooling the data by treatment group. Prior analyses showed no significant interaction between treatment arm and history of diabetes or presence of metabolic syndrome (8). Statistical analyses were performed at the Data Coordinating Center (Axio Research, LLC; Seattle, Washington). All analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). For all analyses, a two-tailed p<0.05 was required for significance.
Results
Baseline Clinical and Laboratory Characteristics
As most study participants by trial design, had low baseline levels of HDL-C and elevated TG levels, the great majority of trial participants already met these two MS criteria at entry. A few who were on statins at entry had HDL-C of 41–42 mg/dL for males and 51–52 mg/dL for females. Similarly, 2,053 of 3,209 participants on statins at entry had TG>150 mg/dL. Among the 3,414 trial patients, 636 patients (19%) had no MS (0 to 2 MS criteria) and 2,741 (81%) were classified as having MS, of whom, 349 (11%) had 3 MS components, 1,094 (32%) had 4 MS characteristics, and 1,298 (38%) had all five components. (Table 1) The other components of the MS cluster were increased waist circumference, present in 2,222 subjects (81%), history of hypertension or BP ≥ 130/85 in 2,146 (78%), and an abnormal blood glucose level or DM in 2,063 (75%).
Table 1.
Baseline Characteristics by Number of Metabolic Syndrome Criteria Met
| No Metabolic Syndrome (2 or fewer criteria) (N=636) |
3 Criteria (N=349) |
4 Criteria (N=1094) |
5 Criteria (N=1298) |
P-value(1) | P-value(2) | ||
|---|---|---|---|---|---|---|---|
| Randomized Treatment Assignment | Monotherapy | 335 (52.7%) | 183 (52.4%) | 524 (47.9%) | 633 (48.8%) | 0.085 | 0.334 |
| Combination Therapy | 301 (47.3%) | 166 (47.6%) | 570 (52.1%) | 665 (51.2%) | |||
| Demographics, baseline physical exam and laboratory measures: | |||||||
| Age | N | 636 | 349 | 1094 | 1298 | 0.052 | <.001* |
| Mean (Std Dev) | 63.1 (9.2) | 62.6 (9.3) | 63.4 (8.6) | 64.5 (8.4) | |||
| Gender | Female | 75 (11.8%) | 46 (13.2%) | 152 (13.9%) | 230 (17.7%) | 0.015* | 0.015* |
| Male | 561 (88.2%) | 303 (86.8%) | 942 (86.1%) | 1068 (82.3%) | |||
| Race | American Indian/Alaska Native/Aboriginal Canadian | 1 (0.2%) | 1 (0.3%) | 4 (0.4%) | 15 (1.2%) | 0.186 | 0.002* |
| Asian | 9 (1.4%) | 10 (2.9%) | 15 (1.4%) | 6 (0.5%) | |||
| Black/African American | 21 (3.3%) | 6 (1.7%) | 40 (3.7%) | 49 (3.8%) | |||
| Native Hawaiian or Other Pacific Islander | 4 (0.6%) | 1 (0.3%) | 1 (0.1%) | 6 (0.5%) | |||
| White | 593 (93.2%) | 323 (92.6%) | 1008 (92.1%) | 1192 (91.8%) | |||
| Muti-racial or Other | 8 (1.3%) | 8 (2.3%) | 25 (2.3%) | 30 (2.3%) | |||
| Ethnicity | Not Hispanic or Latino | 621 (97.6%) | 339 (97.1%) | 1053 (96.3%) | 1224 (94.3%) | 0.013* | 0.025* |
| Hispanic or Latino | 15 (2.4%) | 10 (2.9%) | 41 (3.7%) | 73 (5.6%) | |||
| Systolic blood pressure (mmHg) | N | 636 | 349 | 1093 | 1297 | <.001* | <.001* |
| Mean (Std Dev) | 121.7 (15.1) | 128.1 (15.4) | 128.6 (16.1) | 131.1 (16.4) | |||
| Median (25th, 75th%-tile) | 120.0 (110.0, 129.0) | 128.0 (117.0, 138.0) | 128.0 (118.0, 139.0) | 130.0 (120.0, 141.0) | |||
| Diastolic blood pressure (mmHg) | N | 636 | 349 | 1093 | 1297 | <.001* | 0.224 |
| Mean (Std Dev) | 71.8 (9.5) | 75.7 (9.4) | 75.0 (9.4) | 74.7 (10.1) | |||
| Median (25th, 75th%-tile) | 71.0 (67.0, 78.0) | 76.0 (70.0, 82.0) | 74.0 (70.0, 80.0) | 75.0 (68.0, 80.0) | |||
| Body Mass Index (kg/m2) | N | 635 | 349 | 1091 | 1297 | <.001* | <.001* |
| Mean (Std Dev) | 27.8 (3.9) | 28.7 (4.6) | 31.2 (5.1) | 33.6 (5.1) | |||
| Median (25th, 75th%-tile) | 27.0 (25.0, 30.0) | 28.0 (26.0, 30.0) | 30.0 (28.0, 34.0) | 33.0 (30.0, 36.0) | |||
| Waist circumference (inches) | N | 630 | 348 | 1093 | 1296 | <.001* | <.001* |
| Mean (Std Dev) | 38.1 (4.1) | 39.7 (4.4) | 42.4 (4.9) | 45.1 (4.6) | |||
| Median (25th, 75th%-tile) | 38.0 (35.8, 40.0) | 39.0 (37.0, 42.0) | 42.0 (39.0, 45.7) | 44.1 (42.0, 48.0) | |||
| Glucose (mg/dL) | N | 636 | 346 | 1094 | 1298 | <.001* | <.001* |
| Mean (Std Dev) | 96.9 (10.5) | 99.0 (16.4) | 110.3 (21.9) | 120.9 (23.7) | |||
| Median (25th, 75th%-tile) | 97.0 (91.0, 103.0) | 96.0 (91.0, 99.0) | 104.5 (95.0, 123.0) | 116.0 (105.0, 134.0) | |||
| Insulin (mg/dL) | N | 635 | 348 | 1090 | 1294 | <.001* | <.001* |
| Mean (Std Dev) | 13.3 (13.4) | 14.7 (11.3) | 18.8 (18.4) | 26.1 (35.0) | |||
| Median (25th, 75th%-tile) | 10.2 (7.0, 15.6) | 12.6 (7.9, 18.5) | 14.4 (9.3, 22.5) | 18.3 (12.2, 28.6) | |||
| HOMA1-IR | N | 635 | 345 | 1090 | 1294 | <.001* | <.001* |
| Mean (Std Dev) | 3.2 (3.3) | 3.6 (2.9) | 5.2 (5.5) | 7.9 (11.1) | |||
| Median (25th, 75th%-tile) | 2.4 (1.7, 3.8) | 2.9 (1.9, 4.5) | 3.8 (2.4, 6.0) | 5.4 (3.4, 8.5) | |||
| History of diabetes | 64 (10.1%) | 27 (7.7%) | 320 (29.3%) | 744 (57.3%) | <.001* | <.001* | |
Comparison between no metabolic syndrome and any number of criteria
Comparison among 3, 4, or 5 criteria met
Comparing participants with versus without MS, there was no significant difference in age or assignment to treatment group. The group with MS had a higher proportion of females, Hispanics, and those receiving statin therapy at entry, higher mean blood pressure, body mass index or waist circumference, fasting blood glucose levels, as well as a higher prevalence of DM, and higher percentage of medication use, such as beta-blockers, and inhibitors of the renin-angiotensin system. (Table 1) There was a higher prevalence of prior myocardial infarction, cerebrovascular disease, and peripheral vascular disease among participants with MS. (Table 1)
Comparison among participants with MS according to the number of MS components showed that those who had all 5 components were slightly older, more likely African American, and had significantly higher mean systolic blood pressure, body mass index and waist circumference, fasting blood glucose levels, insulin levels and HOMA1-IR, as well as a higher prevalence of DM, and higher percentage of medication use, including beta-blockers, and inhibitors of the renin-angiotensin system (Table 1). The distribution of prior myocardial infarction, prior percutaneous coronary intervention, history of prior coronary artery bypass graft surgery, cerebrovascular disease or peripheral vascular disease was comparable among all three subgroups. (Table 1)
Comparison of the lipid profile in participants without MS at baseline to those with MS showed no difference in LDL-C levels, lower TG, non-HDL-C, apolipoprotein-B levels and higher HDL-C, lipoprotein(a) and apolipoprotein A levels. (Table 2) Among patients with MS, the subgroup with 5 MS components showed significantly lower LDL-C, TG, non-HDL-C, and apolipoprotein-B levels than those with 3 or 4 components subgroup. (Table 2) The mean LDL-C ranged from the lowest 72.5 mg/dL in the subgroup with 5 MS components to 77 mg/dL in the group with 3 MS components.
Table 2.
Baseline Lipids by Number of Metabolic Syndrome Criteria at Baseline
| No Metabolic Syndrome (2 or fewer criteria) (N=636) |
3 Criteria (N=349) |
4 Criteria (N=1094) |
5 Criteria (N=1298) |
P-value(1) | P-value(2) | ||
|---|---|---|---|---|---|---|---|
| LDL-C (mg/dL) | Mean (Std Dev) | 73.8 (18.0) | 77.2 (27.5) | 75.0 (23.6) | 72.5 (23.2) | 0.123 | 0.004* |
| HDL-C (mg/dL) | Mean (Std Dev) | 36.4 (5.4) | 34.4 (5.1) | 34.5 (5.5) | 34.2 (5.8) | <.001* | 0.466 |
| Triglycerides (mg/dL) | Mean (Std Dev) | 138.2 (40.1) | 203.8 (61.2) | 189.6 (67.6) | 192.5 (69.2) | <.001* | <.001* |
| non-HDL Cholesterol (mg/dL) | Mean (Std Dev) | 101.4 (19.8) | 117.8 (29.4) | 112.9 (27.8) | 110.9 (27.0) | <.001* | <.001* |
| Lp(a) (mg/dL) | Mean (Std Dev) | 83.8 (95.0) | 79.8 (88.9) | 77.8 (89.7) | 70.7 (84.2) | 0.021* | 0.118 |
| ApoA (mg/dL) | Mean (Std Dev) | 124.8 (16.9) | 123.5 (15.6) | 123.2 (15.7) | 122.0 (16.4) | 0.005* | 0.100 |
| ApoB (mg/dL) | Mean (Std Dev) | 78.0 (16.4) | 87.6 (25.6) | 84.5 (20.2) | 83.0 (20.5) | <.001* | 0.002* |
Comparison between no metabolic syndrome and any number of criteria by ANOVA for LDL-C and non-parametric test for other parameters
Comparison among 3, 4, or 5 criteria met
Clinical Outcomes
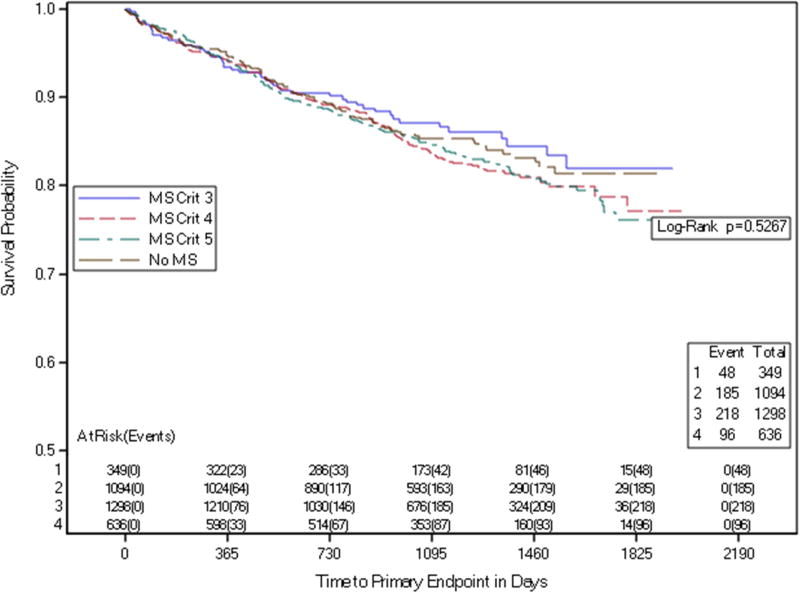
The composite primary endpoint occurred in 15% of participants without MS (0–2 components), in 13.8% of those with 3 MS criteria, in 16.9% of those with 4 MS components), and in 16.8% in those with 5 MS components, P=0.55. (Table 3a) For secondary endpoints, there were no significant differences among subgroups of participants without MS versus those with 3, 4, or 5 components of the MS cluster for the composite endpoint of CV death, myocardial infarction, or stroke, nor for the 4-component endpoint of CV death, myocardial infarction, stroke, or hospitalization for acute coronary syndrome (Table 3a). Similarly, no significant difference in outcomes was demonstrated by Kaplan-Maier analysis among subgroups with 0–2, 3, 4 or 5 components (Figure 1).
Table 3.
| a: Relationship between the number of Metabolic Syndrome Criteria Met at Baseline and Primary/Secondary Endpoints ≤2 Criteria (No Metabolic Syndrome) Referent | ||||
|---|---|---|---|---|
| Number of Metabolic syndrome criteria met at baseline |
Event Number (%) |
HR1 (95%CI), p- value2 |
||
| Primary Endpoint | ≤2 | 96 (15.1%) | Referent, 0.5472 | |
| 3 | 48 (13.8%) | 0.923 (0.653, 1.305) | ||
| 4 | 185 (16.9%) | 1.113 (0.870, 1.425) | ||
| 5 | 218 (16.8%) | 1.115 (0.876, 1.418) | ||
| Secondary Endpoints | . | |||
| Constellation of CAD death, non-fatal MI, ischemic stroke or hospitalization for ACS | ≤2 | 62 (9.7%) | Referent, 0.2726 | |
| 3 | 22 (6.3%) | 0.645 (0.397, 1.050) | ||
| 4 | 109 (10.0%) | 1.000 (0.732, 1.366) | ||
| 5 | 129 (9.9%) | 0.996 (0.736, 1.350) | ||
| Constellation of CAD death, non-fatal MI or ischemic stroke | ≤2 | 55 (8.6%) | Referent, 0.4160 | |
| 3 | 21 (6.0%) | 0.697 (0.422, 1.153) | ||
| 4 | 101 (9.2%) | 1.040 (0.749, 1.445) | ||
| 5 | 111 (8.6%) | 0.960 (0.694, 1.327) | ||
| Cardiovascular Death | ≤2 | 12 (1.9%) | Referent, 0.2594 | |
| 3 | 5 (1.4%) | 0.756 (0.266, 2.147) | ||
| 4 | 28 (2.6%) | 1.301 (0.661, 2.561) | ||
| 5 | 42 (3.2%) | 1.602 (0.843, 3.044) | ||
| Components of the Primary Endpoint | CAD Death | ≤2 | 6 (0.9%) | Referent, 0.3998 |
| 3 | 2 (0.6%) | 0.618 (0.125, 3.062) | ||
| 4 | 18 (1.6%) | 1.752 (0.695, 4.417) | ||
| 5 | 19 (1.5%) | 1.503 (0.599, 3.768) | ||
| Nonfatal MI | ≤2 | 36 (5.7%) | Referent, 0.4349 | |
| 3 | 12 (3.4%) | 0.618 (0.322, 1.188) | ||
| 4 | 61 (5.6%) | 0.976 (0.646, 1.474) | ||
| 5 | 62 (4.8%) | 0.845 (0.560, 1.276) | ||
| Ischemic Stroke | ≤2 | 10 (1.6%) | Referent, 0.5215 | |
| 3 | 5 (1.4%) | 0.910 (0.311, 2.664) | ||
| 4 | 14 (1.3%) | 0.767 (0.340, 1.728) | ||
| 5 | 12 (0.9%) | 0.544 (0.235, 1.260) | ||
| Coronary Events3 | ≤2 | 83 (13.1%) | Referent, 0.3196 | |
| 3 | 46 (13.2%) | 1.027 (0.716, 1.472) | ||
| 4 | 167 (15.3%) | 1.166 (0.896, 1.517) | ||
| 5 | 207 (15.9%) | 1.245 (0.964, 1.607) | ||
| b: Relationship between the number of Metabolic Syndrome Criteria Met at Baseline and Primary/Secondary Endpoints 3 Criteria Referent | ||||
|---|---|---|---|---|
| Number of Metabolic syndrome criteria met at baseline |
Event Number (%) |
HR1 (95%CI), p- value2 |
||
| Primary Endpoint | 3 | 48 (13.8%) | Referent, 0.4467 | |
| 4 | 185 (16.9%) | 1.210 (0.881, 1.663) | ||
| 5 | 218 (16.8%) | 1.217 (0.890, 1.665) | ||
| Secondary Endpoints | Constellation of CAD death, non-fatal MI, ischemic stroke or hospitalization for ACS | 3 | 22 (6.3%) | Referent, 0.1433 |
| 4 | 109 (10.0%) | 1.552 (0.981, 2.454) | ||
| 5 | 129 (9.9%) | 1.553 (0.987, 2.442) | ||
| Constellation of CAD death, non-fatal MI or ischemic stroke | 3 | 21 (6.0%) | Referent, 0.2435 | |
| 4 | 101 (9.2%) | 1.496 (0.935, 2.395) | ||
| 5 | 111 (8.6%) | 1.384 (0.867, 2.208) | ||
| Cardiovascular death | 3 | 5 (1.4%) | Referent, 0.2319 | |
| 4 | 28 (2.6%) | 1.712 (0.661, 4.436) | ||
| 5 | 42 (3.2%) | 2.138 (0.845, 5.407) | ||
| Components of the Primary Endpoint | CAD Death | 3 | 2 (0.6%) | Referent, 0.3687 |
| 4 | 18 (1.6%) | 2.861 (0.664, 12.330) | ||
| 5 | 19 (1.5%) | 2.505 (0.583, 10.767) | ||
| Nonfatal MI | 3 | 12 (3.4%) | Referent, 0.3184 | |
| 4 | 61 (5.6%) | 1.589 (0.855, 2.951) | ||
| 5 | 62 (4.8%) | 1.379 (0.742, 2.562) | ||
| Ischemic Stroke | 3 | 5 (1.4%) | Referent, 0.5469 | |
| 4 | 14 (1.3%) | 0.847 (0.305, 2.352) | ||
| 5 | 12 (0.9%) | 0.600 (0.211, 1.704) | ||
| Coronary Events3 | 3 | 46 (13.2%) | Referent, 0.4417 | |
| 4 | 167 (15.3%) | 1.140 (0.822, 1.580) | ||
| 5 | 207 (15.9%) | 1.223 (0.888, 1.686) | ||
Cox regression models adjusted for treatment arm, sex and age. The number of criteria met as categorical term (HR comparing 3 vs 2, 4 vs. 2, 5 vs. 2).
p-values were calculated based on the Wald test.
Coronary events consist of death from coronary heart disease, non-fatal myocardial infarction, and hospitalization for acute coronary syndrome and coronary revascularization.
Cox regression models adjusted from treatment arm, sex, age. The number of criteria met as categorical term (HR comparing 4 vs. 3, 5 vs. 3).
p-values were calculated based on the Wald test.
Coronary events consist of death from coronary heart disease, non-fatal myocardial disease, hospitalization for acute coronary syndrome or coronary revascularization.
Figure 1. Time to Primary Endpoint by Number of Metabolic Syndrome Components.
Kaplan-Meier curves depicting time to primary endpoint stratified by the number of metabolic syndrome components at baseline.
MS Crit = number of metabolic syndrome criteria
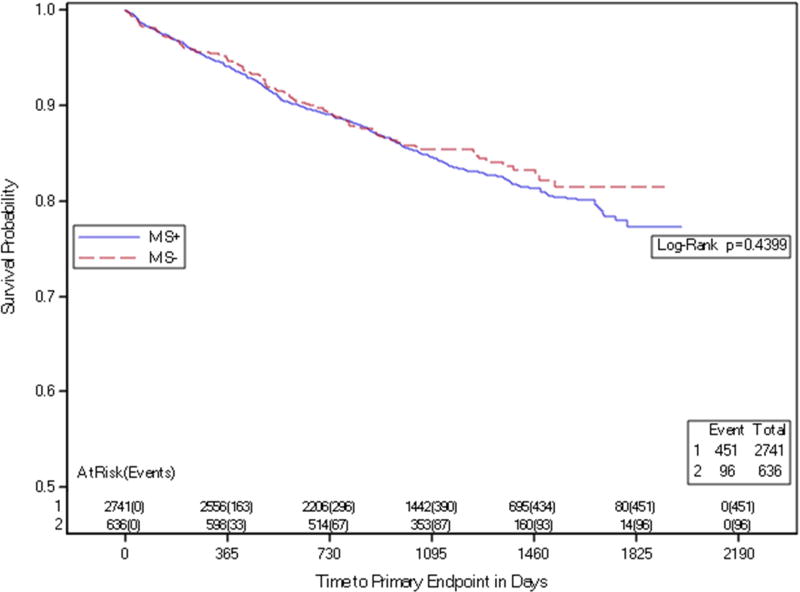
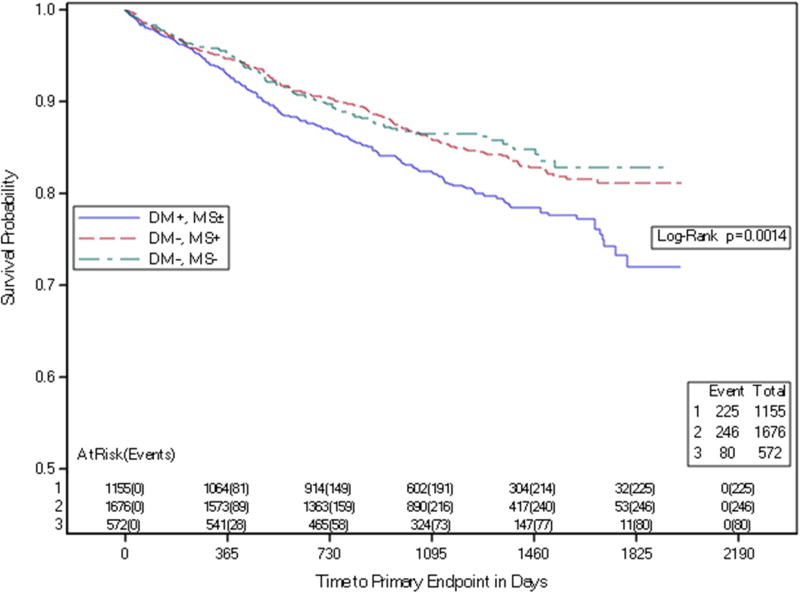
The presence of MS was not associated with increased risk of the composite primary endpoint, pre-specified secondary endpoints or components of the primary endpoint (Table 3a and Figure 2). Among those with MS, a comparison among subgroups with 3, 4 or 5 MS components demonstrated no significant difference in either the composite primary event rate or secondary outcomes, including coronary events only (Table 3b). Diabetes, but not MS cluster, was associated with higher event rates, HR 1.23 (95% CI 1.08–1.39, P=0.001). (Table 4, Figure 3).
Figure 2. Metabolic Syndrome and Primary Endpoint.
Kaplan-Meier curves depicting time to primary endpoint by the presence of metabolic syndrome.
MS+ participants with metabolic syndrome at baseline
MS− participants without metabolic syndrome at baseline
Table 4.
Association of Risk Gradient with Primary and Coronary Endpoints
| Risk Gradient | Event Number (%) |
HR1 (95%CI), p- value2 |
|
|---|---|---|---|
| Primary Endpoint | DM−, MS− | 80 (14.0%) | Referent, 0.0018 |
| DM−, MS+ | 246 (14.7%) | 1.049 (0.815, 1.350) | |
| DM+, MS+/MS− | 225 (19.5%) | 1.411 (1.093, 1.823) | |
| DM+, MS+/MS− vs DM−, MS+/MS− | 1.226 (1.083, 1.388) | ||
| ---, 0.0013 | |||
| Coronary Events | DM−, MS− | 70 (12.2%) | Referent, 0.0005 |
| DM−, MS+ | 226 (13.5%) | 1.108 (0.847, 1.448) | |
| DM+, MS+/MS− | 210 (18.2%) | 1.527 (1.164, 2.004) | |
| DM+, MS+/MS− vs DM−, MS+/MS− | 1.273 (1.118, 1.450) | ||
| ---, 0.0003 |
DM = history of diabetes at baseline, negative (−) or positive (+)
MS = metabolic syndrome at baseline, negative (−) or positive (+)
Cox regression models adjusted for sex, age and the number of criteria met as categorical term. Hazard ratio comparing to DM−, MS− as referent
p-value based on Wald test
Coronary events consist of death from coronary artery disease, myocardial infarction, hospitalization for acute coronary syndrome and coronary revascularization
Figure 3. Time to Primary Endpoint by Risk Gradient.
Kaplan-Meier estimates of time to primary endpoint in participants stratified by risk gradient
DM = history of diabetes mellitus at baseline, negative (−) or positive (+)
MS=metabolic syndrome at baseline, negative (−) or positive (+)
Finally, among the 636 patients without MS at baseline, there was no significant difference in the rate of subsequent progression to MS between those treated with ERN (16.6%) as compared with placebo (15.5%) during a 36-month follow-up (P=0.79).
Discussion
There are several important findings that emerge from this secondary analysis of the AIM-HIGH Trial. First, among enrolled participants with atherogenic dyslipidemia, the prevalence of MS was very high (~80% of all participants). Second, for both the trial primary endpoint and secondary endpoints, clinical outcomes did not differ significantly between those with or without MS at baseline. Third, among participants with MS, there was no significant difference in CV events among the subgroups with 3 components of MS versus 4 or 5 MS components. Furthermore, the presence of DM with or without MS was associated with worse clinical outcomes. Finally, among subjects without features of MS at baseline, randomization to ERN did not appear to affect the subsequent rate of developing MS during a mean 3-year follow-up as compared with those who received placebo. Based on these observations, it appears that neither the presence of MS cluster, nor the number of MS components (3, 4, or 5) provides any incremental prognostic value in this population with stable CV disease and atherogenic dyslipidemia.
In contrast to our findings, the previously-published post hoc analysis of the COURAGE trial of 2,287 subjects showed an increased risk of cardiac events as the number of MS components increased.(4) In COURAGE, the LDL-C levels were higher than in AIM-HIGH, while the longer duration of follow up (5 years) versus a 3-year follow up in AIM-HIGH could have affected the power for the outcomes we observed. Unlike COURAGE participants were not pre-selected based on a particular lipid profile at trial entry, all participants in AIM-HIGH had atherogenic dyslipidemia and low levels of HDL-C and elevated TG at baseline. Those factors might also contribute to the dissimilarity in findings between the two studies.
Previous studies have shown variable and sometimes conflicting findings on the importance of MS on CV event rates in CAD patients. In a study by Solymoss and colleagues, patients with CAD and MS who were followed for a mean of 12 years had higher stroke rates compared to those without MS, but after adjusting for diabetes, there was no significant difference in CV events.(12) A similar observation was noted in the COURAGE post hoc analysis of MS, which was associated with worse outcomes for death or myocardial infarction in univariate analysis (HR=1.4,P=0.001), but not after adjusting for its individual components, (adjusted HR=1.15, P=0.46).(4) Similarly, in a prior study of patients with acute myocardial infarction, no difference in cardiac outcomes was observed among those who underwent percutaneous coronary intervention (PCI) according to presence of MS.(13) However, there have been discordant observations from other studies, showing an association between MS and worse clinical outcomes. In the GISSI-Prevenzione trial, patients with history of prior myocardial infarction and MS had a higher probability of death and CV events.(14) Additionally, in patients with multivessel coronary artery disease enrolled in the Medicine, Angioplasty or Surgery Study and followed for 2 years, the presence of MS was associated with increased mortality, even after adjustment for diabetes.(15) By contrast, however, Patsa et al reported that the presence of MS in patients with proximal left anterior descending coronary disease who underwent PCI was associated with lower incidence of cardiac events during a 20 month mean follow up, with a HR 0.34, p<0.03.(16) Those observations, in aggregate, mitigate the importance of MS as an independent prognostic factor in patients with established CV disease. Identifying MS does not appear to be useful for further risk stratifying statin-treated patients with CV disease. It is possible that the risk inherent in the study population due to the presence of established CV disease overwhelmed any small potential incremental risk brought by the presence of MS. Per recent 2016 ACC Expert Consensus Decision Pathway for non-statin therapies, patients with MS should stay in the clinical atherosclerotic cardiovascular disease without comorbidities pathway unless they have DM.(17) Accordingly, our analysis has demonstrated that DM was a significant predictor of worse CV outcomes independent of the presence of MS.
Because niacin decreases levels of TG, LDL-C, and lipoprotein (a), as well as increasing HDL-C levels, one might infer that niacin should decrease the rate of development of MS. The AIM-HIGH trial clearly showed that ERN increased HDL-C and decreased TG by 25% and 30%, respectively.(7) However, our analysis showed that in AIM-HIGH participants without MS at baseline, ERN did not affect the rate of subsequent MS. This could be explained by the previously mentioned unfavorable effect of niacin on insulin resistance and the potential concern that developing DM on ERN might have offset the positive effect on lipids. Recent AIM-HIGH data demonstrate that ERN increased development of DM and impaired fasting glucose. (19)
Limitations
This was a post hoc analysis of a clinical trial, which has the potential for inherent selection bias as well reduced power to demonstrate difference. Exclusion of patients with acute coronary syndrome or those with poorly-controlled diabetes could affect the results as well. The study population comprised patients with stable CV disease with low HDL and elevated TG, so our observations cannot be applied to the population with CV disease and optimal levels of HDL and TG. Furthermore, it is possible that the very high use of prior statin therapy in AIM-HIGH (75% of patients were taking a statin for 1 or more years prior to randomization and 40% were taking statins for 5 or more years) altered our ability to demonstrate a significant effect of MS on clinical outcomes during the 3-year follow period.
Conclusion
We could not demonstrate that the MS cluster provided prognostic value in patients with established and treated CV disease in this post hoc analysis of the AIM-HIGH trial. Furthermore, the rate of CV events in AIM-HIGH participants with MS was not significantly influenced by the number of MS components. However, the presence of diabetes in AIM-HIGH, with or without MS, was associated with a higher rate of CV events.
Highlights.
Presence of MS did not affect progression of ASCVD.
Number of MS components did not predict CV outcomes in established ASCVD.
DM but not MS was associated with higher events in patients with established ASCVD.
Acknowledgments
AIM-HIGH was supported by the National Heart, Lung, and Blood Institute (U01 HL081616 and U01 HL081649) and by an unrestricted grant from AbbVie, Inc. AbbVie donated the extended release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data.
Financial Disclosure
Dr. Robinson has received research grants to Institution from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, Glaxo-Smith Kline, Merck, Pfizer, Regeneron/Sanofi, Takeda and she is a consultant at: Akcea/Isis, Amgen, Eli Lilly, Esperion, Merck, Pfizer, Regeneron/Sanofi. Dr. Brown has received research grant support from Amgen, Inc. and serves as site principal investigator for a clinical trial funded by Omthera Pharmaceuticals and Astra Zeneca. Dr. Abramson has received honoraria from various pharmaceutical companies including: Amgen, Astra Zeneca, BoehringerIngelheim, Bristol Myer Squibb, Dupont, Eli Lilly, Norvartis, Fournier, Merck Frosst, Pfizer, Servier, Sanofi-Aventis (not speaking beaureau). She also has received funds for ongoing research from Astra Zeneca and Sanofi, and she is on National Advisory Board for Sanofi-Aventis and Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health, the Department of Health and Human Services, or the Department of Veterans Affairs.
Author contributions: Dr. Lyubarova, as first author, drafted and revised the manuscript and lead the effort in these analyses. Drs. Robinson, Miller, Simmons, Abramson, Elam, Ayenew and Brown were collaborating investigator for the AIM-HIGH study and provided scientific input on the content of the manuscript, reviewed and provided suggested revisions to the approach for the analyses. Ms. Xu was the lead statistician for the manuscript, assisted by Ms. McBride, Co-Director for the Data Coordinating Center for AIM-HIGH. Drs. Fleg and Desvigne-Nickens were project officers from NHLBI for AIM-HIGH, members of the Executive Committee and provided scientific input to the manuscript. Dr. Boden is principal investigator for AIM-HIGH and provided scientific input to the manuscript. Drs. Fleg and Boden and Ms. McBride assisted Dr. Lyubarova in writing and revising the manuscript. The manuscript was reviewed and approved by the AIM-HIGH Publications Committee.
All other authors have reported that they have no relationship relevant to the contents of this paper.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, et al. American Heart Association. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015 May 19;313(19):1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 4.Maron DJ, Boden WE, Spertus JA, Hartigan PM, Mancini GBJ, Sedlis SP, et al. Impact of metabolic syndrome and diabetes on prognosis and outcomes with early percutaneous coronary intervention in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol. 2011 Jul 5;58(2):131–7. doi: 10.1016/j.jacc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care. 2015 Jan 1;38(Supplement_1):S49–57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 7.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013 May;34(17):1279–91. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011 Dec 15;365(24):2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 9.AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011 Mar;161(3):471–7.e2. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beilby J. Definition of Metabolic Syndrome: report of National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment:insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Solymoss BC, Bourassa MG, Marcil M, Levesque S, Varga S, Campeau L. Long-term rates of cardiovascular events in patients with the metabolic syndrome according to severity of coronary-angiographic alterations. Coron Artery Dis. 2009 Jan;20(1):1–8. doi: 10.1097/MCA.0b013e32831624a5. [DOI] [PubMed] [Google Scholar]
- 13.Won K-B, Kim B-K, Chang H-J, Shin D-H, Kim J-S, Ko Y-G, et al. Metabolic syndrome does not impact long-term survival in patients with acute myocardial infarction after successful percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2014 Apr 1;83(5):713–20. doi: 10.1002/ccd.25150. [DOI] [PubMed] [Google Scholar]
- 14.Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, et al. Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005 Jul 19;46(2):277–83. doi: 10.1016/j.jacc.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Lopes NH, Paulitsch FS, Pereira AC, Gois AF, Gagliardi A, Garzillo CL, et al. Impact of metabolic syndrome on the outcome of patients with stable coronary artery disease: 2-year follow-up of the MASS II study. Coron Artery Dis. 2008 Sep;19(6):383–8. doi: 10.1097/MCA.0b013e328306aa8a. [DOI] [PubMed] [Google Scholar]
- 16.Patsa C, Toutouzas K, Tsiamis E, Tsioufis C, Spanos A, Karanasos A, et al. Impact of metabolic syndrome on clinical outcomes after new generation drug-eluting stent implantation: the “obesity paradox” phenomenon is still apparent. Nutr Metab Cardiovasc Dis NMCD. 2013 Apr;23(4):307–13. doi: 10.1016/j.numecd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Writing Committee. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC., Jr 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016 Jul 5;68(1):92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg RB, Bittner VA, Dunbar RL, Fleg JL, Grunberger G, Guyton JR, Leiter LA, McBride R, Robinson JG, Simmons DDL, Wysham C, Xu P, Boden WE. Effects of Extended Release Niacin added to Simvastatin/Ezetemibe on Glucose and Insulin values in AIM HIGH. Am J Med. 2016 Jul;129(7):753, e13–22. doi: 10.1016/j.amjmed.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]