Figure 1.
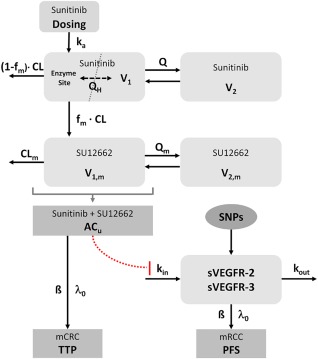
Model structure. ACU, unbound active concentration (sunitinib + SU12662); β, regression coefficient; CLm, clearance of the metabolite SU12262; fm, fraction metabolized to SU12662; ka, absorption rate constant; kin, zero‐order release rate constant; kout, first‐order elimination rate constant; λ0, baseline hazard; Q, inter‐compartmental clearance of sunitinib; QH, liver blood flow; Qm, intercompartmental clearance of the metabolite SU12662; V1, volume of the central compartment of sunitinib; V1,m, volume of the central compartment of the metabolite SU12662; V2, volume of the peripheral compartment of sunitinib; V2,m volume of the peripheral compartment of the metabolite SU12662.
