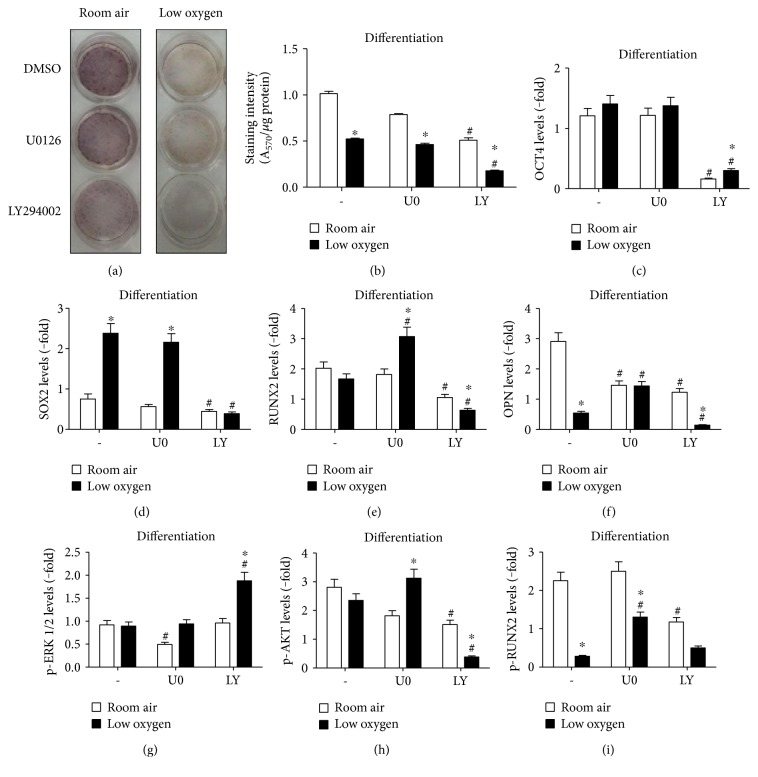
Figure 7.
PMSC differentiation is mediated via MEK1/2 and PI3K signaling and their inhibition effect on ERK1/2, AKT, and RUNX2 phosphorylation under low oxygen tension. PMSCs were cultured for 14 days in osteogenic differentiation conditions containing 2% FBS in room air (20% O2) or low oxygen levels (1% O2). During the 14 days, cells were continuously exposed to (5 μM) U0126 or (10 μM) LY294002 in differentiation media. Treatments were stopped at 14 days and stained with alizarin red to confirm (a) PMSC differentiation morphology changes with the inhibitors and quantified in (b) (two-way ANOVA, P < 0.05, N = 4). Immunoblots, shown in Figure S7, were used to quantify protein levels of (c) OCT4, (d) SOX2, (e) RUNX2, (f) OPN, (g) p-ERK1/2, (h) p-AKT, and (i) p-RUNX2 induced by signaling inhibition. Quantification levels were normalized to β-actin, a protein loading control; additionally, each phosphoprotein was normalized to its total protein (two-way ANOVA, P < 0.05, N = 3). ∗ indicates significance between room air and low oxygen tension; # indicates significance between DMSO control and inhibitor.