Abstract
Case summary
A 5-year-old male neutered domestic shorthair cat was referred with a history of persistent pyrexia, pica, soft faeces, inappetence, intermittent vomiting, mild-to-moderate granulocytosis and mild hypercalcaemia. No significant improvement was noted after antibiotic and corticosteroid treatment, except that the hypercalcaemia resolved. Physical examination, including thoracic auscultation, and abdominal and peripheral lymph node palpation, were unremarkable. On admission, haematology revealed moderate leukocytosis (36.8 × 109/l) with moderate-to-marked eosinophilia (21.3 × 109/l) and marked basophilia (4.04 × 109/l), the latter identified microscopically. Lymphocytes were markedly decreased (0.37 × 109/l). Blood smear examination revealed 58% eosinophils, 28% neutrophils, 11% basophils, 2% monocytes, 1% lymphocytes and marked, diffuse platelet clumping. Biochemistry abnormalities indicated mild pancreatitis, dehydration and anorexia with mildly increased pancreatic lipase, mild hypernatraemia (157 mmol/l), a moderate decrease in urea (3.1 mmol/l) and a slight decrease in phosphate (1.32 mmol/l). Ultrasound and radiographic imaging revealed enlargement of the mesenteric lymph nodes. Fine-needle aspiration, a Tru-cut biopsy and immunohistochemistry were performed. Cytological examination revealed ~65–75% lymphocytes (~80% were larger than a neutrophil), ~25–35% eosinophils and occasional basophils. Lymphocytes had single, small (<1/3 red blood cells), prominent nucleoli and increased pale, mildly vacuolated cytoplasm. On histopathology, cells were monomorphic, large, with prominent nucleoli, and mild, multifocal, staining for T-cell marker CD3. Smaller cells were strongly CD3-positive. Cells were negative for B-cell marker CD45R.
Relevance and novel information
This is the most severe case of paraneoplastic basophilia reported with feline alimentary T-cell lymphoma with accompanying eosinophilia and lymph node infiltration. Feline basophil prevalence is reported for the first time.
Case description
A 5-year-old male neutered domestic shorthair cat was referred to the University College Dublin Veterinary Hospital (UCDVH) with a 6 week history of lethargy, inappetence and pica. Pyrexia of 1 month’s duration, a recent history of soft faeces and intermittent vomiting were also reported. No improvement was noted after antibiotic and corticosteroid treatment, and the patient was referred for further investigations. On physical examination the cat was quiet, alert and responsive, with a body condition score of 5/5 and weighing 7.6 kg. The mucous membranes were pink with a capillary refill time <2 s. Thoracic auscultation and abdominal palpation were unremarkable. Rectal temperature was 40.1°C and the peripheral lymph nodes were normal.
Salient blood results from both the referring veterinarian (LaserCyte; IDEXX Laboratories) and on admission to UCDVH (Advia 2120; Siemens Healthcare Diagnostics) revealed persistent eosinophilia and basophilia, with values significantly increased at the time of referral (21.32 × 109/l and 4.04 × 109/l, respectively). Erythron and platelet values indicated borderline anaemia and marked thrombocytopenia. Mild hypercalcaemia was identified before referral, whereas urea was moderately decreased before and after admission. While hospitalized, feline pancreatic-like immunoreactivity test (IDEXX Laboratories) revealed pancreatitis (5.7 µg/l; reference interval [RI] 0.1–3.5 µg/l). Urine culture revealed no growth and the patient was negative for Toxoplasma gondii, feline immunodeficiency virus/feline leukaemia virus and Giardia species infection. Table 1 summarises the haematology and biochemistry data obtained from the referring veterinary practice and on admission to UCDVH.
Table 1.
Haematology and plasma biochemistry panel from the referring veterinarian and on admission to University College Dublin Veterinary Hospital (UCDVH)
| Parameter | Referring practice | Reference interval | UCDVH | Reference interval |
|---|---|---|---|---|
| Haematology | ||||
| Haematocrit (l/l) | 0.27 | 0.30–0.45 | 0.26 | 0.24–0.45 |
| Haemoglobin (g/l) | 97 | 90–151 | 86 | 81–142 |
| RBCs (× 1012/l) | 5.7 | 5–10 | 5.4 | 5–10 |
| MCH (pg) | 17 | 12–20 | 15.9 | 12–16 |
| MCHC (g/l) | 356 | 290–375 | 334 | 300–360 |
| MCV (fl) | 47.7 | 41–58 | 47.7 | 39–55 |
| Platelets (× 109/l) | >200 | 175–600 | 53 | 180–550 |
| MPV (fl) | 5.5 | – | 22.7 | 8.6–18.9 |
| WBCs (× 109/l) | 51.9 | 5.5–19.5 | 36.8 | 6–18 |
| Segmented neutrophils | 39.8 | 2.5–12.5 | 10.3 | 2.5–12.5 |
| Band neutrophils | – | – | 0.0 | 0.0–0.0 |
| Lymphocytes | 1.37 | 0.4–6.8 | 0.37 | 1.5–7 |
| Monocytes | 8.13 | 0.15–1.7 | 0.74 | 0.04–0.85 |
| Eosinophils | 2.4 | 0.1–0.79 | 21.3 | 0–1.5 |
| Basophils | 0.2 | 0.0–0.1 | 4.04 | 0–0.04 |
| PT (s) | – | – | 18 | 9–14 |
| aPTT (s) | – | – | 16.8 | 10–15 |
| Biochemistry | ||||
| Total protein (g/l) | 70 | 57–89 | 71.1 | 59–78 |
| Albumin (g/l) | 29 | 22–40 | 33.7 | 25–35 |
| Globulin (g/l) | 41 | 28–51 | 37.4 | 24–40 |
| Calcium (mmol/l) | 2.98 | 1.95–2.83 | 2.4 | 1.5–3.3 |
| Urea (mmol/l) | 3.9 | 5.7–12.9 | 3.1 | 6.6–10 |
| Creatinine (µmol/l) | 115 | 71–212 | 98 | 40–170 |
| Phosphate (mmol/l) | 1.84 | 1.0–2.42 | 1.32 | 1.4–2.5 |
| GLDH (U/l) | – | – | 16 | 0–17 |
| ALT (U/l) | 32 | 12–130 | 31 | 0–45 |
| Sodium (mmol/l) | – | – | 157.1 | 147–156 |
| Chloride (mmol/l) | – | – | 117.2 | 108–122 |
| Potassium (mmol/l) | – | – | 3.96 | 4–4.5 |
| Cobalamin (ng/l) | – | – | >1000 | ⩾275 |
| Folate (µg/l) | – | – | 12.8 | 8.2–13.5 |
RBCs = red blood cells; MCH = mean cell haemoglobin; MCHC = mean cell haemoglobin concentration; MCV = mean cell volume; MPV = mean platelet volume; WBCs = white blood cells; PT = prothrombin time; aPTT = activated partial thromboplastin time; GLDH = glutamate dehydrogenase; ALT = alanine aminotransferase
Microscopic examination of the blood smear revealed a differential count of 58% eosinophils, 28% neutrophils, 11% basophils, 2% monocytes and 1% lymphocytes (Figure 1). Basophils were identified exclusively by routine microscopic examination of the smear. They were occasionally larger than an eosinophil, with a ribbon-like, sometimes bi- or tri-lobed, and rarely U-shaped nucleus with moderately condensed chromatin and numerous relatively uniform, round, lavender cytoplasmic granules. Basophil morphology was compared with that of healthy cats and was found to be unremarkable, with no significant differences in granule size, number and staining, or differences in nuclear segmentation or chromatin pattern. More than 95% of basophils were well granulated with prominent, individual granules (Figure 2, inset), rarely with peripheralised or poorly stained granules (Figure 2). Also, there were large numbers of well-granulated eosinophils with segmented nuclei and abundant orange, rod-shaped granules. Rare eosinophilic metamyelocytes were observed (not shown). Marked and diffuse platelet clumping was also noted throughout the smear area.
Figure 1.
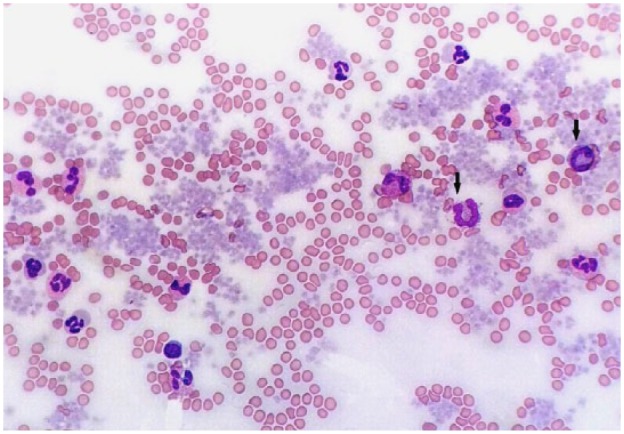
Blood smear. Numerous granulocytes, of which two are basophils (arrows), and marked, diffuse platelet clumping. Objective × 40. Romanowsky stain
Figure 2.
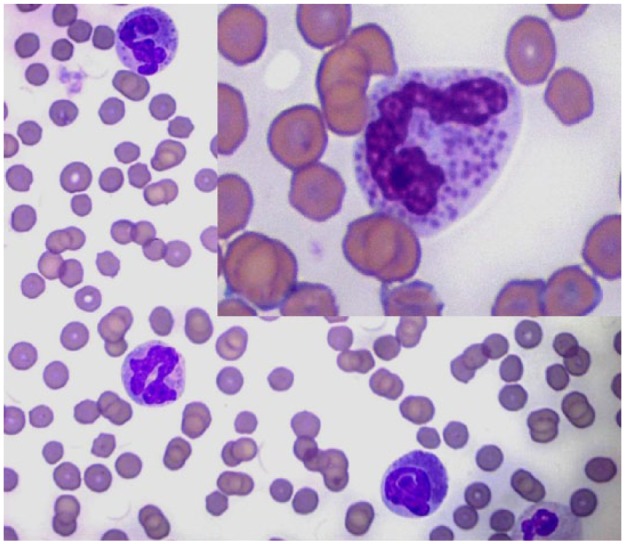
Blood smear. Three feline basophils. Inset: detail of the cytoplasmic granules. Objective × 100. Romanowsky stain
Further tests included radiographic imaging and abdominal ultrasound, both of which identified a mid-abdominal mass consistent with markedly enlarged mesenteric lymph nodes. Samples for cytological evaluation were obtained by fine-needle aspiration (FNA) from the mesenteric and sternal lymph nodes and spleen, and submitted for analysis. The mesenteric lymph node (Figures 3 and 4) was highly cellular with good cell preservation in a haemodiluted background. Approximately one-third, occasionally up to half, of nucleated cells were well-granulated eosinophils, markedly out of proportion to the blood contamination (Figure 3). Occasional basophils were noted (Figures 3 and 4). The lymphoid population consisted of mostly (>80%) medium-sized lymphocytes (2–2.5 red blood cells [RBCs] in diameter) with round-to-oval, often irregular, indented and off-centre nuclei, fine chromatin and usually single small (<1/3 RBCs) and prominent nucleoli (Figure 4). Occasionally, lymphocytes were >3 RBCs in diameter. Light blue cytoplasm was in moderate amounts, frequently with small, clear vacuoles. Large macrophages with phagocytosed cellular debris or intact eosinophils were occasionally noted. The cytological features of the FNA of the sternal lymph node were similar to that of the mesenteric, with a lower cell yield and a lower eosinophilic infiltration (20%). There was a mixed population of lymphocytes with one-third to half being medium-sized, as described previously. Occasional basophils, rare eosinophilic metamyelocytes and rare mast cells were observed. The splenic aspirate revealed frequent eosinophils (25%) and basophils (10%) in a background of fresh blood. The lymphoid population was mixed, with mostly small lymphocytes (<1.5 RBCs in diameter), whereas one-third had nuclear diameters larger than two RBCs. Frequent metarubricytes, rubricytes, rare rubriblasts and rare eosinophilic metamyelocytes were also noted. The cytological diagnosis was lymphoma of both mesenteric and sternal lymph nodes with marked and moderate eosinophilic infiltration, respectively, mild basophilic infiltration for both, and accompanying moderate extramedullary haematopoiesis of the spleen.
Figure 3.
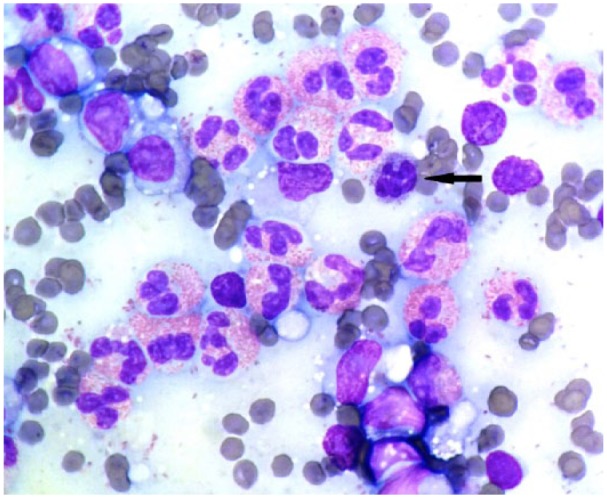
Photomicrograph of the mesenteric lymph node. Marked eosinophilic infiltration, one basophil (arrow) and medium-sized lymphocytes with prominent nucleoli. Objective × 100. Romanowsky stain
Figure 4.
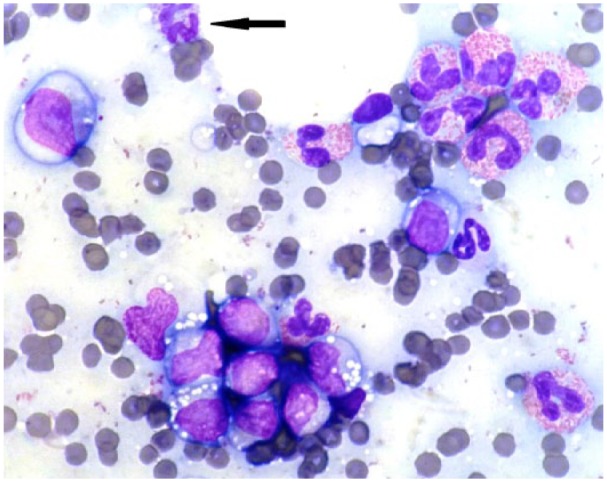
Photomicrograph of the mesenteric lymph node. Medium-sized and large lymphocytes with irregular, indented nuclei, fine chromatin pattern and increased, pale blue cytoplasm with small, clear vacuoles. Frequent eosinophils and one basophil observed (arrow). Objective × 100. Romanowsky stain
To further characterise the tumour, immunocytochemistry and polymerase antigen receptor rearrangement tests were performed. While both examinations proved inconclusive owing to insufficient material recovered, a faint band suggestive of a clonal population was noted on the electropherogram. A Tru-cut biopsy of the mesenteric lymph node was later submitted for analysis. Prior to sampling, repeat coagulation values remained mildly prolonged.
Histopathological examination revealed a diffuse infiltrate of round cells admixed with large numbers of intact, degranulated eosinophils (Figure 5). Morphologic similarities with the cytological sample included a monomorphic population of round cells with large, round-to-ovoid nuclei with prominent nucleoli and stippled chromatin. The cells typically exhibited finely granular, pale-basophilic to clear cytoplasm, anisocytosis and occasional nuclear hyperchromasia. Mitotic activity was minimal (<1 per 10 high-power fields). Immunohistochemistry (University of Liverpool, Veterinary Laboratory Services) revealed smaller diameter lymphocytes to be strongly positive for CD3, a pan T-cell marker, whereas the larger lymphocytes were diffusely and multifocally mildly positive. Both the small lymphocytes and the neoplastic cells were positive for major histocompatibility complex II antigen, a macrophage and dendritic lineage marker, whereas the latter were negative for CD45R, a specific pan-B-cell marker for cats. Toluidine blue staining and CD117 (C-kit), mast cell markers, were negative, ruling out a visceral mastocytoma. A final diagnosis of alimentary T-cell lymphoma was made.
Figure 5.
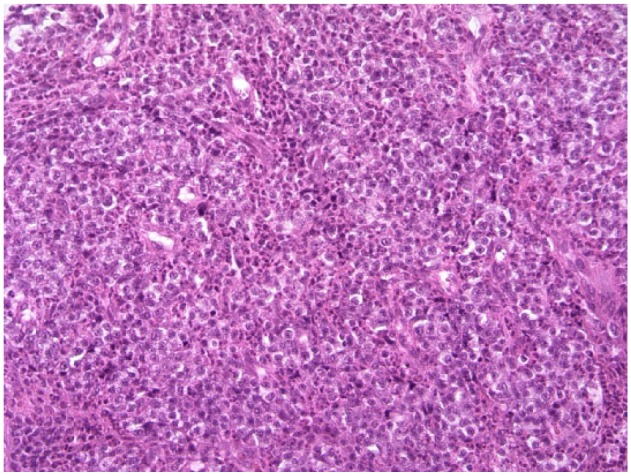
Tru-cut section of the mesenteric lymph node. Diffuse infiltrate of round cells and frequent eosinophils. Objective × 40. Hematoxylin and eosin stain
While hospitalised, the cat received fluid therapy and remained alert with a body temperature below 40°C. There was no evidence of bleeding from the biopsy sites. Unfortunately, after discharge and a mild episode of anorexia at home, the owner opted for euthanasia, which was carried out by the referring veterinarian. The remains were not available for post-mortem examination.
Discussion
Feline paraneoplastic basophilia has rarely been documented.1–5 Non-neoplastic causes of basophilia are primarily attributed to allergic reactions and parasitism, previously reported with heartworm infection and polycythemia vera.1,5,6 Other inflammatory disorders such as food allergy, flea dermatitis, eosinophilic granuloma and asthma are observed during IgE-mediated hypersensitivity reactions, and typically accompany eosinophilia.6,7 The main cytokines responsible for the production and proliferation of basophils are interleukin (IL)-3 and granulocyte macrophage colony-stimulating factor, whereas IL-4 is the major cytokine responsible for tissue infiltration.6
In cats, paraneoplastic basophilia has previously been reported with intestinal and epitheliotropic lymphoma of T-cell origin,1,2 feline large granular lymphocyte lymphoma,8 acute and chronic myelogenous leukaemia,3,4 and malignant mesenchymal tumour.5
Lymphoma is one of the most common neoplasms reported in cats.9 In this patient, the cytological diagnosis was based on the proportion and size of the lymphocytes,10 as >50% had irregular, eccentric nuclei exceeding 10 µm in diameter with prominent nucleoli and increased cytoplasm.11 This was confirmed on histopathology, and, coupled with the referring hypercalcaemia, persistent eosinophilia and basophilia, persistent pyrexia, clinical signs, negative staining for CD45R and the positive staining for CD3, was identified as T-cell in origin. Likely due to the steroid treatment, the hypercalcaemia was only identified before admission to UCDVH. The clinical signs and biochemistry results indicated anorexia, mild dehydration and mild pancreatitis, and the thrombocytopenia was artefactual, due to the marked, diffuse aggregates observed microscopically.
In feline patients, lymphoma is located predominantly in the gastrointestinal (GI) tract, where up to 25% are T-cell in origin (CD3+).12,13 It can be associated with mild-to-severe peripheral eosinophilia and eosinophilic infiltrates of the duodenum and the mesenteric lymph nodes.1,2,7,14 Paraneoplastic eosinophilia is typically a feature of mast cell tumours, also reported in cats with myeloproliferative disease, transitional cell carcinoma and lymphoma.2,3 While hypereosinophilia and T-cell lymphoma are well documented in humans, only rare cases have been reported in cats and dogs.14,15 Non-neoplastic eosinophil values can range from 23.5 × 109/l to 46.2 × 109/l, reported in cats with flea allergy and eosinophilic granuloma complex, respectively.16 Values >50 × 109/l are characteristically seen in rare disorders such as idiopathic hypereosinophilic syndrome and eosinophilic leukaemia.16 In this case, the cause of the increased circulating eosinophils was attributed to the confirmed alimentary T-cell lymphoma, whereas other inflammatory conditions were eliminated based on history and clinical signs. Neither eosinophilic nor basophilic leukaemia were suspected in the present case as circulating granulocytes were mature, well differentiated with rare band forms and rare eosinophilic metamyelocytes, and no blast cells were noted during the blood smear examination. Eosinophilic leukaemia is rare in the feline patient,16–18 whereas basophilic leukaemia as a primary neoplasm has been reported exclusively in dogs and humans.19–21
Electronic records from the UCDVH Clinical Pathology laboratory identified only five cases of basophilia from approximately 7000 cats in the past 7 years. Values ranged from 0.6–3.49 × 109/l (RI 0–0.04 × 109/l). In the same time period, only one canine patient was identified with a value of 1.18 × 109/l. Internal records also revealed that basophil relative counts of ⩽1% and absolute values of ⩽0.3 × 109/l have a prevalence of 8–10% in the feline population.
Special consideration should be given to both automated and manual counts, as basophils can often be misinterpreted. When performing manual counts, emphasis should be put on differentiating basophils from monocytes or toxic neutrophils by their cytoplasmic characteristics,22 and from mast cells based on their nuclear morphology.23 In one study, three haematology analysers were evaluated with respect to their ability to detect canine, feline and leporine basophils, considered to be resistant to acid lysis, differentiating them from the rest of the white cell population. Canine basophils were not detected by the Sysmex XT-2000iV or CELL-DYN 3500 analysers,22,24 and neither canine nor feline basophils were identified by the Advia 2120.22 Scattergram cluster interpretation for basophils and eosinophils is predominantly useful in cats for the Sysmex XT-2000iV.5,24
Paraneoplastic basophilia and eosinophilia in cats have been rarely reported together, predominantly with haemolymphatic malignancies, and usually accompanied by clinical signs of GI disease.1–4 Feline basophils had mild-to-moderate increases, ranging from 0.2 × 109/l with chronic myeloid leukaemia,4 0.28 × 109/l with the acute form,3 and up to 0.58 × 109/l with intestinal T-cell lymphoma.2 The highest reported value was 2.8 × 109/l,1 also documented in a case of intestinal lymphoma. Eosinophil counts in these cases were 7.7, 10.7, 2.6 and 4.3 × 109/l, respectively. In dogs, rare papers have reported basophilia with thymoma,25,26 accompanied by lymphocytosis, GI T-cell lymphoma and visceral mastocytoma.27,28 Basophil values ranged from 0.3 and 0.68 to 2.8 × 109/l,26–28 with 4.8 × 109/l being the highest reported value in a dog with probable essential thrombocythemia.29 The eosinophil counts were 2.9, 0.5, 35.8 and 1.0 × 109/l, respectively.
Conclusions
A literature review identified only five feline cases of paraneoplastic peripheral basophilia with associated eosinophilia, two of which were reported with intestinal lymphoma, and one identified as T cell in origin. This is the most severe case of paraneoplasic basophilia reported with feline alimentary T-cell lymphoma with accompanying eosinophilia and lymph node infiltration. Identified exclusively during microscopic evaluation of blood smears, slight basophilia (⩽1% or ⩽0.3 × 109/l) has a prevalence of 8–10% in the feline population. Eosinophilia cases should be manually screened for basophilia, and both relative and absolute basophil increases should prompt suspicion of an underlying lymphoid neoplasm in the feline patient.
Acknowledgments
The authors wish to thank Jeremiah O’Connor and Oaklawns Veterinary Surgery Clinic for their collaboration; Dr Lorenzo Ressel and Valerie Tilston from the University of Liverpool for carrying out and interpreting the immunohistochemistry; ECVIM-CA Diplomate Barbara Gallagher for her management of the case; Brian Cloak for his assistance with the photomicrographs; and all technical staff in the University College Dublin Veterinary Hospital clinical pathology laboratory.
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 15 August 2017
References
- 1. Thorn ET, Aubert I. Abdominal mass aspirate from a cat with eosinophilia and basophilia. Vet Clin Pathol 1999; 28: 139–141. [DOI] [PubMed] [Google Scholar]
- 2. Cave TA, Gault EA, Argyle DJ. Feline epitheliotrophic T-cell lymphoma with paraneoplastic eosinophilia – immunochemotherapy with vinblastine and human recombinant interferon α2b. Vet Comp Oncology 2004; 2: 91–97. [DOI] [PubMed] [Google Scholar]
- 3. Gilroy C, Forzán M, Drew A, et al. Eosinophilia in a cat with acute leukemia. Can Vet J 2011; 52: 1004–1008. [PMC free article] [PubMed] [Google Scholar]
- 4. Mochizuki H, Seki T, Nakahara Y, et al. Chronic myelo-genous leukemia with persistent neutrophilia, eosinophilia and basophilia in a cat. J Feline Med Surg 2014; 16: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stranieri A, Ferrari R, Zanzani S, et al. Sysmex XT-2000iV scattergram analysis in a cat with basophilia. Vet Clin Pathol 2016; 45: 225–228. [DOI] [PubMed] [Google Scholar]
- 6. Pohlman ML. Basophils, mast cells, and their disorders. In: Schalm’s veterinary hematology. 6th ed. Ames, Iowa: Blackwell Publishing, 2010, pp 290–297. [Google Scholar]
- 7. Barrs VR, Beatty JA, McCandlish IA, et al. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J Small Anim Pract 2002; 43: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finotello R, Vasconi ME, Sabattini S, et al. Feline large granular lymphocyte lymphoma: an Italian Society of Veterinary Oncology (SIONCOV) retrospective study. Vet and Comp Oncology. Epub ahead of print 29 May 2017. DOI: 10.1111/vco.12325. [DOI] [PubMed] [Google Scholar]
- 9. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 10. Lynch A, Papakonstantinou S, O’Brien PJ. Quantitative cytomorphometry of Romanowsky-stained smears for the diagnosis of canine lymphoma. Vet Clin Path 2011; 40: 581. [Google Scholar]
- 11. Papakonstantinou S, O’Brien PJ. High content imaging for the morphometric diagnosis and immunophenotypic prognosis of canine lymphomas. Cytometry B Clin Como 2014; 86: 373–382. [DOI] [PubMed] [Google Scholar]
- 12. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 13. Waly NE, Gruffydd-Jones TJ, Stokes CR, et al. Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. J Comp Path 2005; 133: 253–260. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi Y, Takahashi M, Fujino Y, et al. Intestinal T-cell lymphoma with severe hypereosinophilic syndrome in a cat. J Vet Med Sci 2012; 74: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 15. Marchetti V, Benetti C, Citi S, et al. Paraneoplastic hyperosinophilia in a dog with intestinal T-cell lymphoma. Vet Clin Pathol 2005; 34: 259–263. [DOI] [PubMed] [Google Scholar]
- 16. Gelain ME, Antoniazzi E, Bertazzolo W, et al. Chronic eosinophilic leukemia in a cat: cytochemical and immunophenotypical features. Vet Clin Pathol 2006. 35: 454–459. [DOI] [PubMed] [Google Scholar]
- 17. Swenson CL, Carothers MA, Wellman ML, et al. Eosinophilic leukemia in a cat with naturally acquired feline leukemia virus infection. J Am Anim Hosp Assoc 1993; 29: 467–501. [Google Scholar]
- 18. Sharifi H, Nassiri SM, Esmaelli H, et al. Eosinophilic leukaemia in a cat. J Feline Med Surg 2007; 9: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mears EA, Raskin RE, Legendre AM. Basophilic leukemia in a dog. J Vet Intern Med 1997; 11: 92–94. [DOI] [PubMed] [Google Scholar]
- 20. Bernini JC, Timmons CF, Sandler ES. Acute basophilic leukemia in a child. Anaphylactoid reaction and coagulopathy secondary to vincristine-mediated degranulation. Cancer 1995;; 75: 110–114. [DOI] [PubMed] [Google Scholar]
- 21. Pardanani AD, Morice WG, Hoyer JD, et al. Chronic basophilic leukemia: a distinct clinico-pathologic entity? Eur J Haematol 2003; 71: 18–22. [DOI] [PubMed] [Google Scholar]
- 22. Lilliehook I, Tvedten H. Errors in basophil enumeration with 3 veterinary hematology systems and observations on occurrence of basophils in dogs. Vet Clin Pathol 2011; 40: 450–458. [DOI] [PubMed] [Google Scholar]
- 23. Harvey JW. Veterinary hematology. A diagnostic guide and color atlas. St Louis, MO: Elsevier, 2012. [Google Scholar]
- 24. Lilliehook I, Tvedten H. Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. II. Differential leukocyte counts. Vet Clin Pathol 2009; 38: 175–182. [DOI] [PubMed] [Google Scholar]
- 25. Simpson RM, Waters DJ, Gebhard DH, et al. Massive thymoma with medullary differentiation in a dog. Vet Pathol 1992; 29: 416–419. [DOI] [PubMed] [Google Scholar]
- 26. Burton AG, Borjesson DL, Vernau W. Thymoma-associated lymphocytosis in a dog. Vet Clin Pathol 2014; 43: 584–588. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Fowlkes N, Evans ED, et al. What is your diagnosis? Abdominal fluid from a dog. Vet Clin Pathol 2013; 42: 113–114. [DOI] [PubMed] [Google Scholar]
- 28. Cowgill E, Neel J. Pleural fluid from a dog with marked eosinophilia. Vet Clin Pathol 2003; 32: 147–149. [DOI] [PubMed] [Google Scholar]
- 29. Hopper PE, Mandell CP, Turrel JM, et al. Probable essential thrombocythemia in a dog. J Vet Intern Med 1989; 3: 79–85. [DOI] [PubMed] [Google Scholar]


