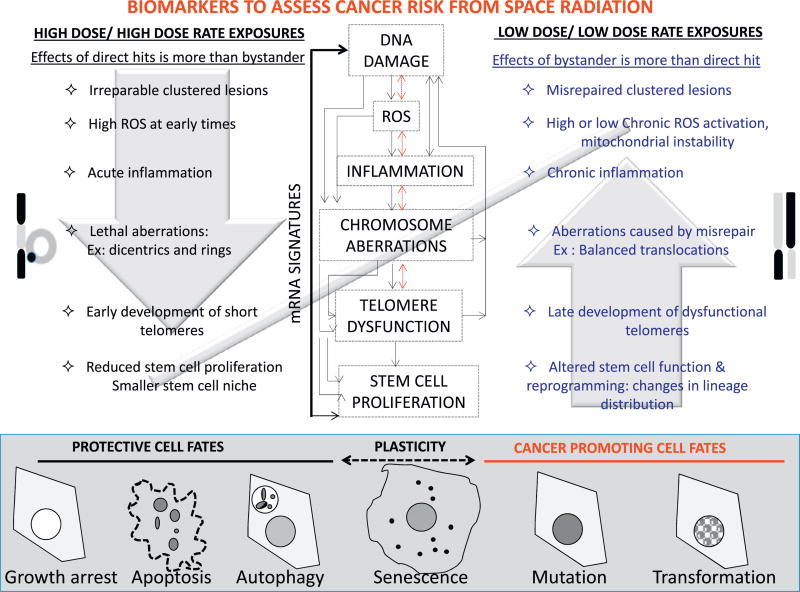
Fig. 1. Schematic representation of known mechanistic links between biomarkers that define cell fates, which promote or protect from cancer risk.
We have detailed the feedback network between various biomarkers of cellular stress response at early and late times post radiation exposure. For simplicity biomarkers have been defined as “early” and “late”, although some early biomarkers are also observed at later times. Early biomarkers evaluated include persistent damage, DNA repair foci, alterations in ROS and inflammation. Late biomarkers of genomic instability include mutations, chromosome aberrations and telomere length changes. The phenotype of each of these biomarkers differ post exposure to HD/HDR and LD/LDR radiation. As majority of the current data is based on HD/HDR exposures, biological effects of LD/LDR exposures that mimic GCR are speculative, need to be further validated using GCR simulator at NSRL, and hence are highlighted in blue. Summary of current literature suggests that majority of acute high-dose low-LET exposures cause complex clustered lesions that are irreparable. In contrast, although cells hit with low doses of high-LET radiation also get complex clustered damages due to intense energy deposition along the track, bystander effects appear to play a prominent role in the ensuing biological response, pathways activated and the eventual fate of the cell. Most of the irreparable clustered lesions initiate a cascade of biological processes that are so damaging that the inherent protective mechanisms prevent carcinogenesis by targeting these cells for growth arrest, apoptosis, autophagy or senescence. Cells with fewer numbers of complex clustered lesions and those with non-lethal lesions caused by high-LET radiation may, however, continue to proliferate following misrepair of the lesions. Chromosome aberrations or mutations caused by these lesions perpetuate further DNA damage, chronic ROS, chronic inflammation and telomere dysfunction, resulting in more genomic instability and in some cases cellular transformation. Cell fate choice and distribution may differ between HD/HDR and LD/LDR exposures, and is an essential area that needs further investigation. The nodes of intersection between endpoints that govern the choice between pro- and anti- survival cell fates must be factored in along with temporal mRNA/protein expression profiles, in order to obtain a clear biomarker signature for modeling and early prediction of cancer risk. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)